Pyrazolopyrimidine compound containing piperazine or its medicinal salt and preparation method and application thereof
A technology for pyrazolopyrimidines and compounds is applied in the field of pyrazolopyrimidine compounds or their medicinal salts and their preparation, which can solve the problems such as slowing down the growth rate of tumors and achieve the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
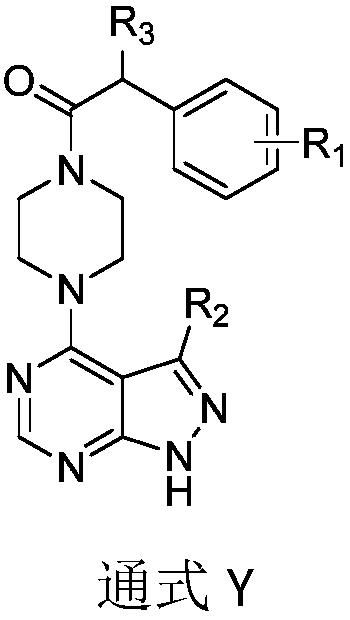
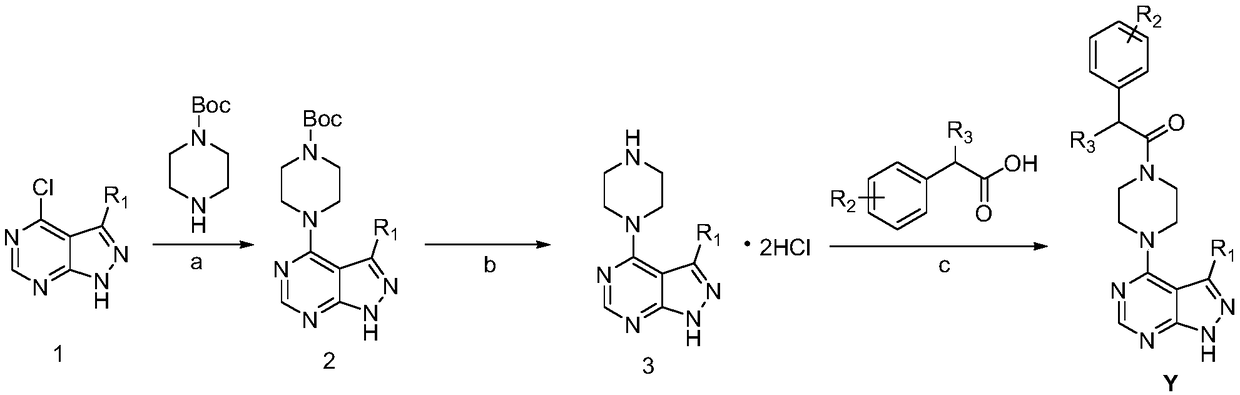
[0049] Preparation of Compound Y
[0050] 1) Preparation of Intermediate 2:
[0051] Dissolve 3-substituted-1H-pyrazolo[3,4-d]pyrimidine (10mmol) in DMF (15mL), add DIEA (15mmol, 2.5mL), 1-tert-butoxycarbonylpiperazine (10.5mmol ), microwave reaction at 90°C for 20-30min, after the reaction was completed, the reaction solution was quenched with ice water (150mL), extracted with ethyl acetate (3×30mL), the organic phases were combined, and saturated ammonium chloride (3 ×20mL), washed the organic phase with saturated brine (3×20mL), dried over anhydrous sodium sulfate, filtered, evaporated the solvent under reduced pressure, purified by column chromatography (petroleum ether: ethyl acetate = 1:3) to obtain intermediate 2 .
[0052] The prepared intermediate 2 is the following compounds:
[0053] tert-butyl 4-(1H-pyrazolo[3,4-d]pyrimidin-4-yl)piperazine-1-carboxylate (2a)
[0054] White solid, 89% yield, 1 H NMR (400MHz, DMSO) δ8.94(s,1H), 8.60(s,1H), 4.32(s,4H), 3.35(s,4H)...
Embodiment 2
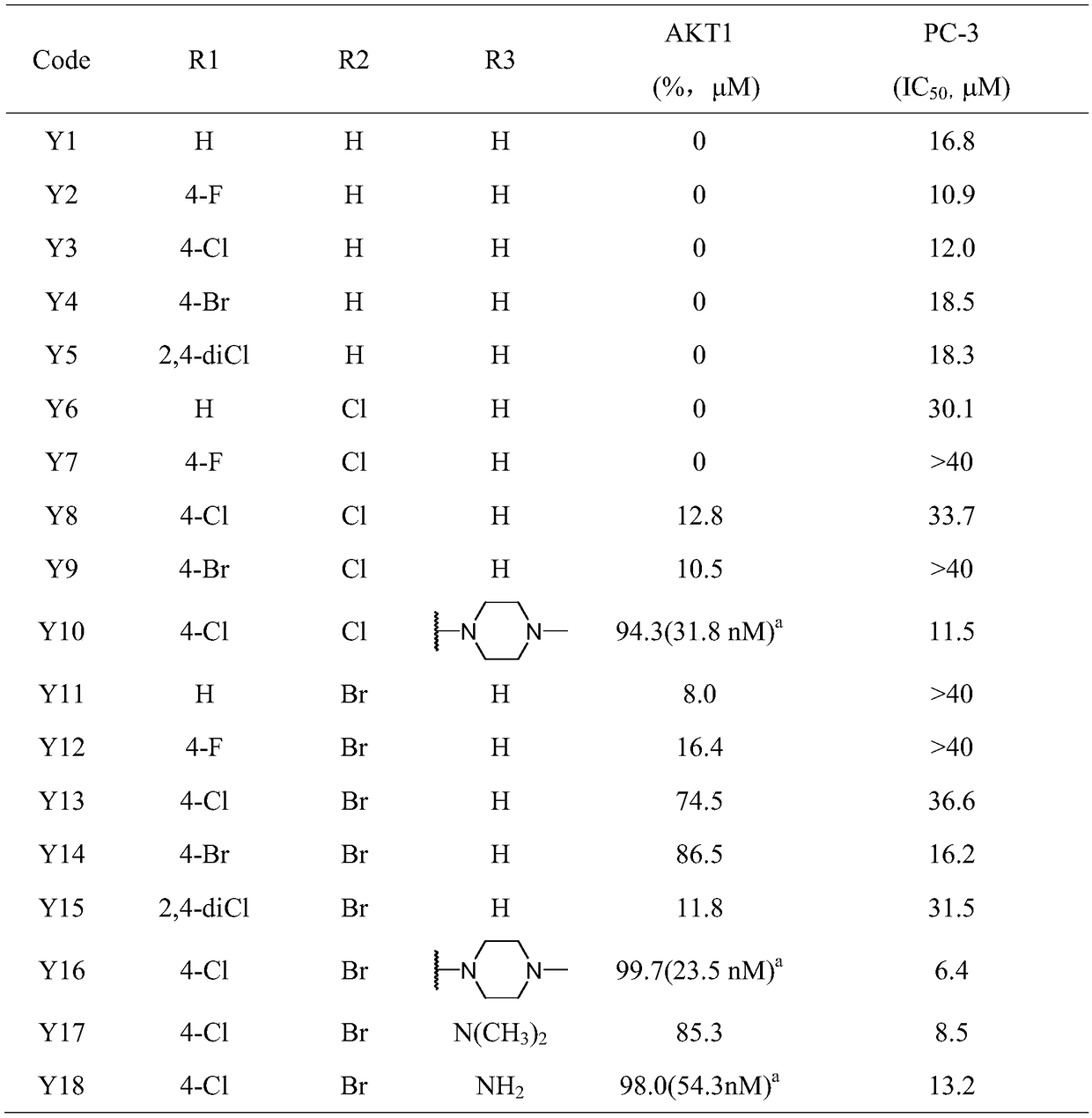
[0107] Determination of compound's inhibitory activity on AKT1 kinase and anti-proliferation activity of PC-3 cells
[0108] The biological activity of the target compounds Y1 to Y18 prepared in Example 1 was determined according to the following method, and the results are shown in Table 1.
[0109] The inhibitory activity of the compound on AKT1 kinase and the anti-proliferation activity of PC-3 cells were carried out using the methods reported in the literature. For details, see: LIu Y, Yin Y, Zhang J, NomIe K, Zhang L, Yang D, Wang ML, ZhaoG.2016. DIscovery of 4-(PIperazIn-1-yl)-7H-pyrrolo[2,3-d]pyrImIdIneDerIvatIves as Akt InhIbItors. Arch Pharm (WeInheIm) 349:356-362. LIu Y, Yin Y, Zhang Z, LI C.J, Zhang H, Zhang D, JIang C, NomIe K, Zhang L, Wang M.L, ZhaoG. 2017. Structural optImIzatIon elaborates novel potent Akt InhIbItors wIthpromIsIng antIcancer actIvIty. Eur J Med Chem 138:543-551.
[0110] Table 1. Target compounds Y1 to Y18 have inhibitory activity on AKT1 kina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com