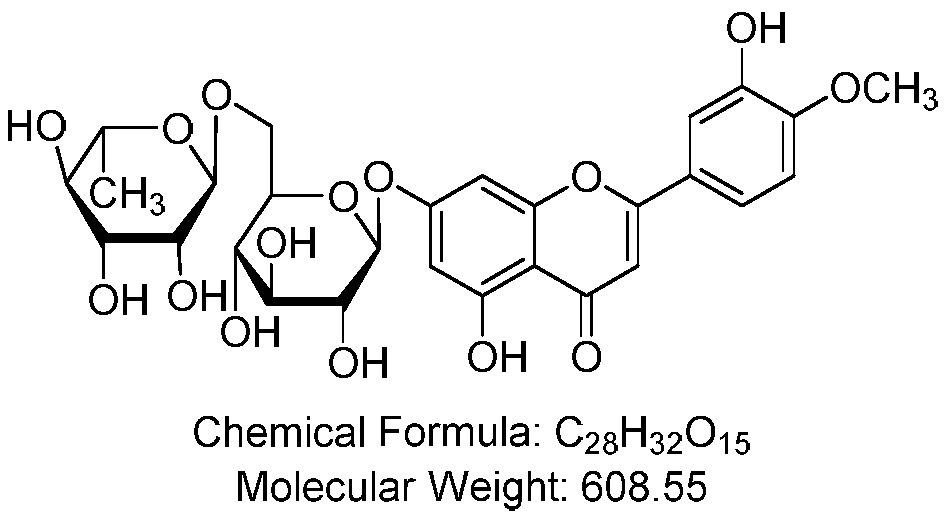
A kind of preparation method of high-purity diosmin
A diosmin, high-purity technology, applied in the field of separation and purification of high-purity drug diosmin, to achieve the effects of less environmental pollution, good selectivity, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Preparation of sample solution: Weigh 1.25 g of crude diosmin, add 50 mL of 0.2M NaOH solution, ultrasonically dissolve and filter for later use.
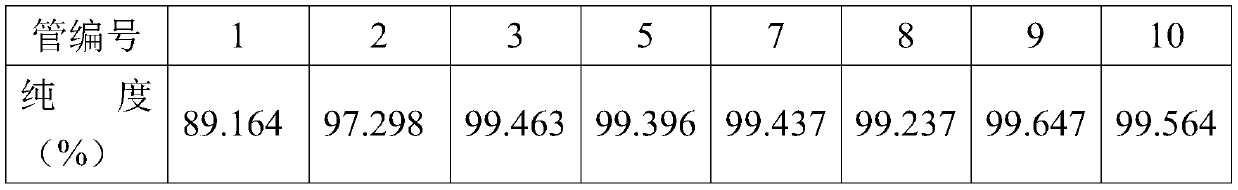
[0033] 2) Chromatographic separation process: the chromatographic filler UniPS TM Pack 10-300 in a 15mm*310mm chromatographic column, wash 200mL with deionized water, then inject the sample prepared in step 1) into the chromatographic column with a flow rate of 10mL / min, continue to use deionized water after loading Rinse until the first miscellaneous peak is gone, discard the eluent. After the baseline of the chromatographic column is stable, use the first eluent to elute until the main peak appears, and then use the second eluent to elute after the main peak has come out. Collect the eluent in 50mL per tube, and collect the eluent according to the time sequence of collection. Each tube was numbered, and the purity of diosmin was tested. The results are as follows:
[0034] Tube number 1 2 3 4 5 7 29 ...
Embodiment 2
[0038] 1) Preparation of sample solution: Weigh 60.0 g of crude diosmin, add 2.4 L of 0.2M NaOH solution, ultrasonically dissolve and filter for later use.
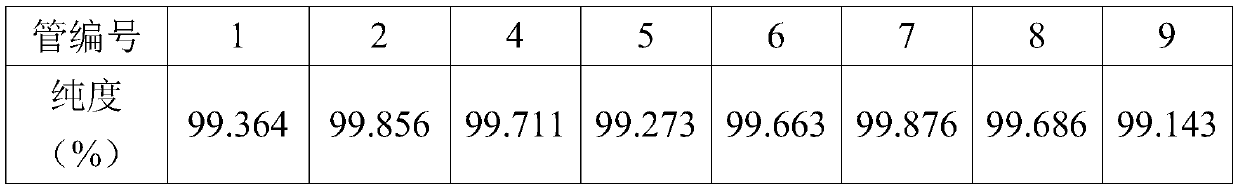
[0039] 2) Chromatographic separation process: the chromatographic filler UniPS TM Pack 10-300 in a 70mm*460mm chromatographic column, wash 4L with deionized water, then inject the sample prepared in step 1) into the chromatographic column with a flow rate of 50mL / min, continue to use deionized water after loading Rinse until the first miscellaneous peak is gone, discard the eluent. After the baseline is stable, use the first eluent to elute until the main peak appears, and then use the second eluent to elute after the main peak comes out. Use 2L of each eluent (numbers 3 to 6 are 1L, and the rest are 2L) ) collection, each tube is numbered according to the time sequence of collection, and the purity of diosmin is detected, the results are as follows:
[0040] Tube number 2 3 4 6 8 10 purity(%) 95.6...
Embodiment 3
[0044] 1) Preparation of sample solution: Weigh 60.0 g of crude diosmin, add 2.4 L of 0.2M NaOH solution, ultrasonically dissolve and filter for later use.
[0045] 2) Chromatographic separation process: the chromatographic filler UniPS TM Pack 10-300 in a 70mm*460mm chromatographic column, wash 4L with deionized water, then inject the sample prepared in step 1) into the chromatographic column with a flow rate of 50mL / min, continue to use deionized water after loading Rinse until the first miscellaneous peak is gone, discard the eluent. After the baseline is stable, use the first eluent to elute until the main peak appears, and then use the second eluent to elute after the main peak comes out. Use 2L of each eluent (numbers 3 to 6 are 1L, and the rest are 2L) ) collection, each tube is numbered according to the time sequence of collection, and the purity of diosmin is detected, the results are as follows:
[0046]
[0047] Then combine the diosmin eluents with a purity g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com