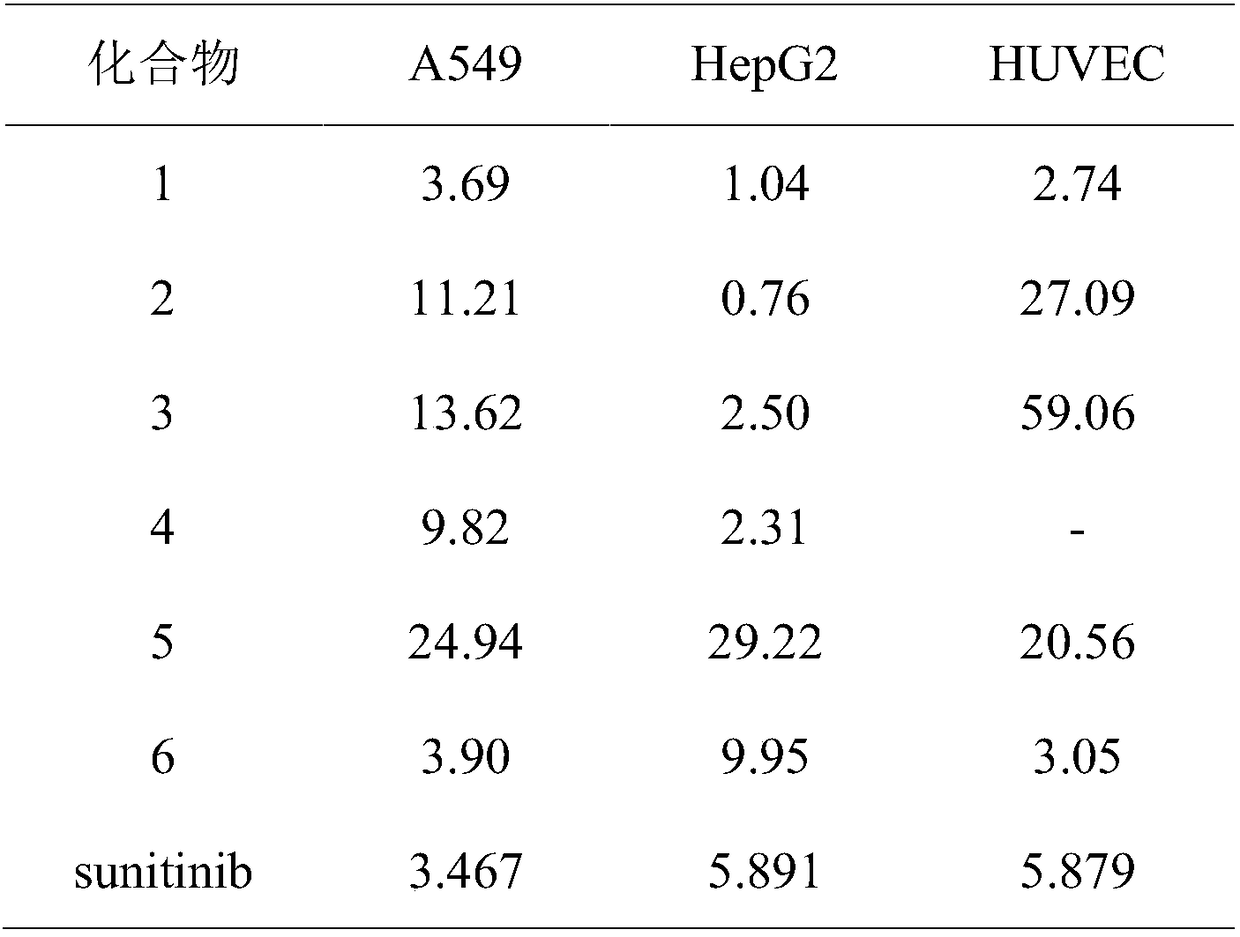
Preparation and application of six PARP1 inhibitors
A technology of inhibitors and excipients, applied in the field of medicine, can solve the problems of no reports of six compounds, and achieve the effects of good industrial production prospects, strong inhibitory activity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
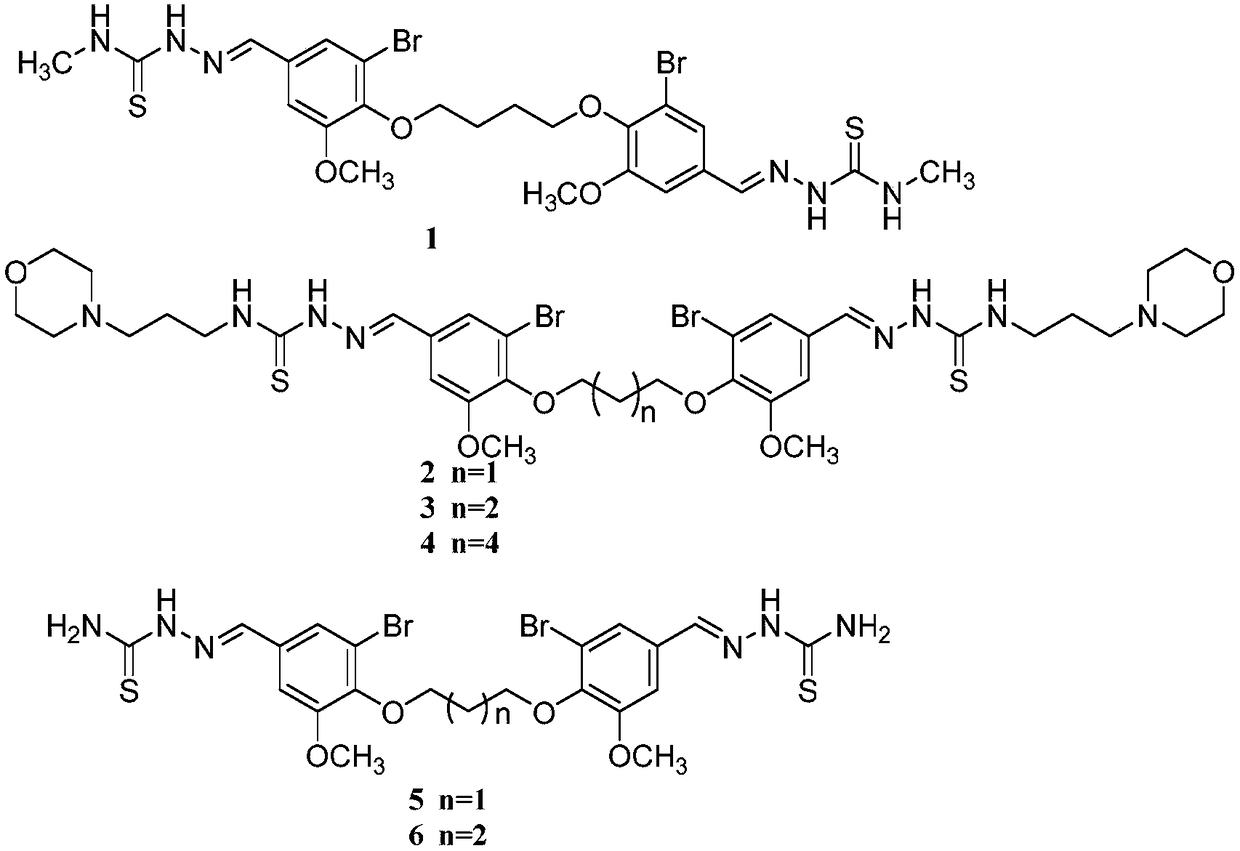
[0040] Example 1: 2,2'-(((butane-1,4-diyldioxy)bis(3-bromo-5-methoxy-4-phenyl))dimethylene)bis(N -methylhydrazine-1-thioamide)(2,2'-((((butane-1,4-diylbis(oxy)) bis(3-bromo-5-methoxy-4, 1-phenylene))bis( methanelylylidene))bis(N-methylhydrazine-1-carbothioamide), compound 1) preparation;
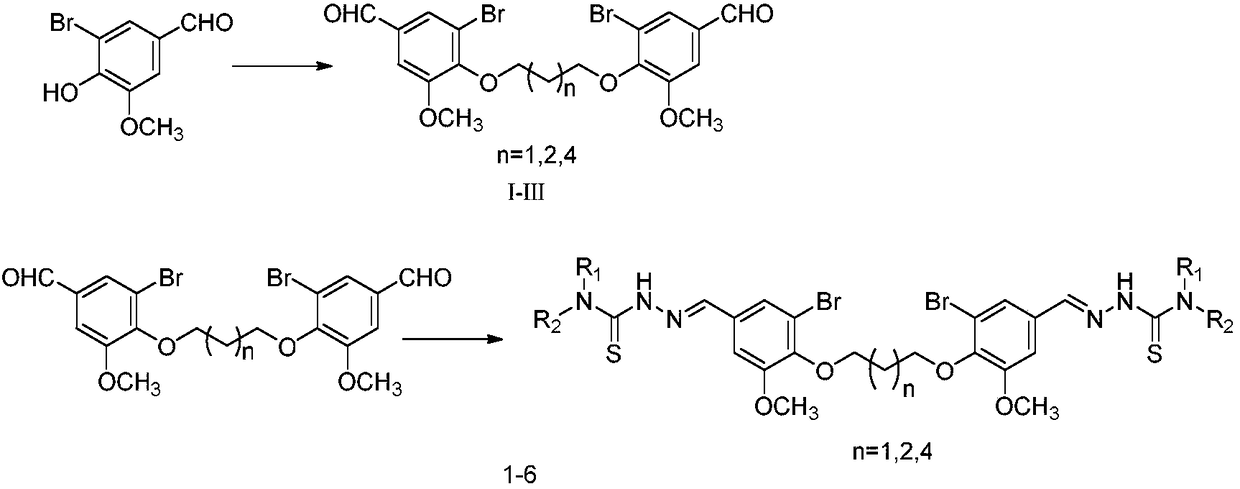
[0041] (1) Weigh 5-bromovanillin (13.9g, 60mmol) and potassium carbonate (2.8g, 20mmol) into a 1L flask, add 20mL of DMF to dissolve, stir for 10min, add 1,4-dibromobutane dropwise while stirring (2mL, 20mmol), heated and condensed at 80°C for reflux for 10h, added 500mL of water, a white flocculent precipitate precipitated, filtered the precipitate, washed the precipitate with saturated sodium bicarbonate, washed the precipitate with a small amount of ethyl acetate, and dried to obtain intermediate compound II .
[0042] (2) Weigh 0.2mmol of intermediate compound II (100mg) and 0.44mmol of 4-methylthiosemicarbazide (46.3mg) into a 100mL reaction flask, add 10mL of 95% ethanol, stir for 10...
Embodiment 2
[0043] Example 2: 2,2'-(((propane-1,3-diyldioxy)bis(3-bromo-5-methoxy-4-phenyl))dimethylene)bis(N- (3-morpholino)hydrazine-1-thioamide)(2,2'-(((propane-1,3-diylbis(oxy))bis (3-bromo-5-methoxy-4,1-phenylene ))bis(methanelylylidene))bis(N-(3-morpholinopropyl)hydrazine-1-carbothioamide),compound 2)
[0044] The preparation method of compound 2 is similar to the preparation method of compound 1, and its difference from example 1 is that the raw material 1,4-dibromobutane is replaced by 1,3-dibromopropane to prepare intermediate compound I, and then By replacing 4-methylaminothio with 4-[3-(4-morpholine)propyl]-3-thiosemicarbazide, a white solid compound 2 was prepared with a yield of 83.2%. 1H-NMR (500MHz, DMSO- d6 )δ: 11.50(s, 2H, CH), 8.56(t, J=6Hz, 2H, NH), 7.94(s, 2H, NH), 7.68(s, 2H, ArH), 7.35(d, J=1.5 Hz, 2H, ArH), 4.19 (t, J=6.5Hz, 4H, OCH2), 3.85 (s, 6H, OCH3), 3.59 (dd, 4H, CH2), 3.54 (s, 8H, CH2), 2.31 ( m, 10H, CH2), 2.12 (m, 2H, CH2), 1.74 (m, 4H, CH2); 13C NMR (1...
Embodiment 3
[0045] Example 3: 2,2'-(((butane-1,4-diyldioxy)bis(3-bromo-5-methoxy-4-phenyl))dimethylene)bis(N -(3-morpholino)hydrazine-1-thioamide)(2,2'-(((butane-1,4-diylbis(oxy))bis (3-bromo-5-methoxy-4,1- phenylene))bis(methanelylidene))bis (N-(3-morpholinopropyl)hydrazine-1-carbothioamide),compound 3)
[0046] The preparation method of compound 3 is similar to the preparation method of compound 1, which is prepared in the same way as in Example 1 to obtain intermediate compound II, and then 4-methylaminosulfur is replaced by 4-[3-(4-morpholine) propyl ]-3-thiosemicarbazide, a white solid compound 3 was prepared with a yield of 84.6%. 1H-NMR (500MHz, DMSO-d 6 )δ: 11.49(s, 2H, CH), 8.55(t, J=6Hz, 2H, NH), 7.94(s, 2H, NH), 7.68(s, 2H, ArH), 7.35(s, 2H, ArH ), 4.02(s, 4H, OCH 2 ), 3.86(s, 6H, OCH 3 ), 3.59 (dd, 4H, CH 2 ), 3.54(s, 6H, CH 2 ), 2.31 (m, 10H, CH 2 ), 1.90(s, 4H, CH 2 ), 1.74 (m, 4H, CH 2 ); 13 C NMR (125MHz, DMSO-d 6 )δ: 177.3, 154.0, 146.3, 140.7, 131.8, 122.9, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com