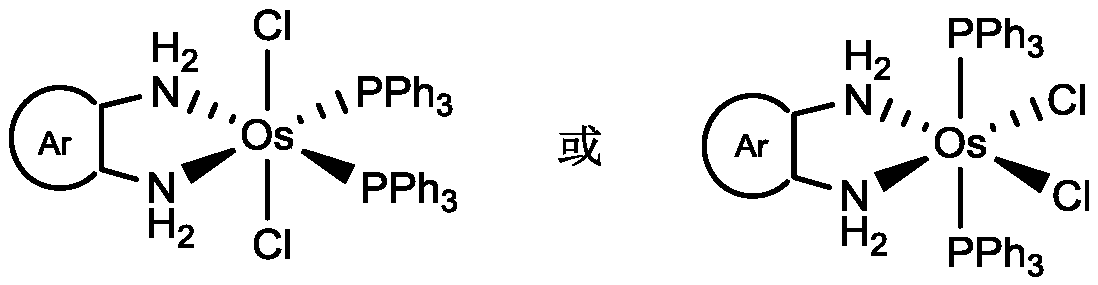
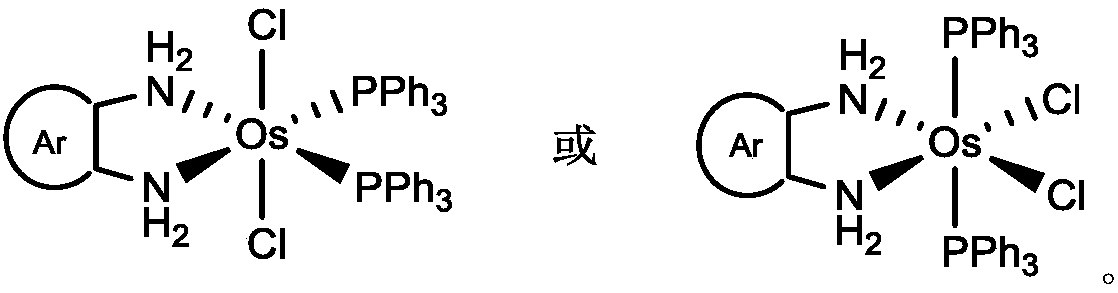
Osmium double-teeth diamine complex with effect of catalyzing dehydrogenation activity of ammonia borane and preparation method thereof
A technology for catalyzing ammonia borane and bidentate diamine, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, organic chemistry, etc. Difficulties and other problems, to achieve the effect of mild reaction conditions, low energy consumption, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh o-phenylenediamine (22mg, 0.20mmol) and OsCl 2 (PPh 3 ) 3 (178mg, 0.17mmol) into 25mL reaction bottle with magnetic sub, under N 2 Under protection, 15 mL of dichloromethane solvent was added, and the reaction was stirred at 20-25° C. for 2 h, and the solution changed from dark green to brown-red. After the reaction was completed, the reaction solution was concentrated, and 20 mL of anhydrous n-hexane was added to form a light yellow precipitate, which was the crude product of o-phenylenediamine-substituted osmium nitrogen complex. The crude product was washed with n-hexane (4 times, 40 mL), filtered and dried to obtain the purified osmium nitrogen complex light yellow solid with a yield of 136 mg and a yield of 90%.
[0026] The results of NMR analysis were 1 H NMR (600MHz, CDCl 3 )δ: 7.4 (broad s, 12H, PPh 3 ), 7.2(t, J=7.2Hz, 6H, PPh 3 ), 7.1(t, J=7.2Hz, 12H, PPh 3 ),7.0(broad s,2H,Ar),6.8(broad s,2H,Ar),5.0(broad s,4H,NH 2 ). 13 C{ 1 H}NMR (151MHz, C...
Embodiment 2
[0029] Weigh 3-methyl-o-phenylenediamine (24mg, 0.20mmol) and OsCl 2 (PPh 3 ) 3 (178mg, 0.17mmol) into 25mL reaction bottle with magnetic sub, under N 2 Under protection, 15 mL of dichloromethane solvent was added, and the reaction was stirred at 20-25° C. for 2 h, and the solution changed from dark green to brown-red. After the reaction was completed, the reaction solution was concentrated, and 20 mL of anhydrous n-hexane was added to form a yellow precipitate, which was the crude product of 3-methyl-o-phenylenediamine substituted osmium nitrogen complex. The crude product was washed with n-hexane (4 times, 40 mL), filtered and dried to obtain the purified osmium nitrogen complex yellow solid, with a yield of 133 mg and a yield of 86%.
[0030] The results of NMR analysis were 1 H NMR (600MHz, CDCl 3 )δ:7.5(q,J=8.8Hz,12H,PPh 3 ), 7.2(t, J=4.4Hz, 6H, PPh 3 ), 7.1(t, J=7.5Hz, 12H, PPh 3 ),6.9(t,J=7.5Hz,1H,Ar),6.8(d,J=3.6Hz,1H,Ar),6.6(d,J=4.5Hz,1H,Ar),5.0(broad s,2H ,NH...
Embodiment 3
[0033] Weigh 3-bromo-o-phenylenediamine (38mg, 0.2mmol) and OsCl 2 (PPh 3 ) 3 (178mg, 0.17mmol) into 25mL reaction bottle with magnetic sub, under N 2 Under protection, 15 mL of dichloromethane solvent was added, and the reaction was stirred at 20-25° C. for 2 h, and the solution changed from dark green to brown-red. After the reaction was completed, the reaction liquid was concentrated, and 20 mL of anhydrous n-hexane was added to form a yellow precipitate, which was the crude product of osmium nitrogen complex substituted by 3-bromo-o-phenylenediamine. The crude product was washed with n-hexane (4 times, 40 mL), filtered and dried to obtain the purified osmium nitrogen complex yellow solid, with a yield of 129 mg and a yield of 78%.
[0034] The results of NMR analysis were 1 H NMR (600MHz, CDCl 3 )δ:7.4(broad s,12H,PPh 3 ),7.3(broad s,1H,Ar),7.2(broad s,PPh 3 ), 7.1 (broad s, 12H, PPh 3 ), 6.9(t, J=6.3Hz, 1H, Ar), 6.8(d, J=7.2Hz, 1H, Ar), 5.1(broad s, 2H, NH 2 ), 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com