Method for preparing niacinamide nucleoside salt
A technology for nicotinamide nucleoside salt and nicotinamide nucleoside trifluoromethanesulfonate, which is applied in the field of preparing nicotinamide nucleoside salt, can solve the problems of reduced total yield, unsuitability for industrial production, increased reduction reaction and the like, and achieves the Simple operation, suitable for large-scale production, avoid the effect of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-A
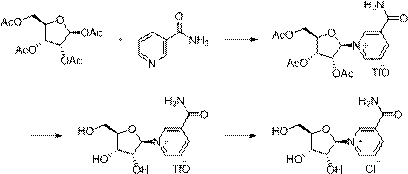
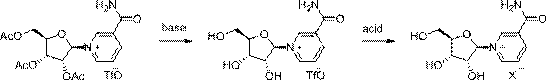
[0033] Embodiment 1-A: Preparation of nicotinamide nucleoside chloride
[0034]A mixture of triacetyl nicotinamide nucleoside trifluoromethanesulfonate (53g, 0.1mol) and methanol (50ml) was added dropwise to 7N ammonia methanol solution (100ml) at -10°C, and kept stirring at -10°C for 24h , after the sampling detection reaction finishes, control -10 ℃ and drop 28% hydrogen chloride methanol solution (100ml) to neutralize, filter to remove ammonium chloride, filtrate control -10 ℃ drop 28% hydrogen chloride methanol solution (40ml) to replace ions, Add ethyl acetate (1000ml) dropwise to crystallize, keep stirring at -10°C for 1h, and filter to obtain the crude product.
[0035] Add methanol (100ml) to the crude product to cool down to -10°C, add dropwise 28% methanolic hydrogen chloride solution (6ml), beat for 4 hours, and filter to obtain a beating solid; % hydrogen chloride methanol solution (3ml), beating for 4h, the beating temperature is -5°C, the wet product was obtaine...
Embodiment 1-B
[0036] Example 1-B: Preparation of nicotinamide nucleoside chloride
[0037] A mixture of triacetyl nicotinamide nucleoside trifluoromethanesulfonate (53g, 0.1mol) and methanol (50ml) was added dropwise to 30% sodium methoxide methanol solution (27g, 0.15mol) at -7°C, and kept at -7 Stir the reaction at ℃ for 1 hour, and after the reaction is completed by sampling and testing, add 28% methanolic hydrogen chloride solution (25ml) dropwise at -4°C to neutralize, filter to remove sodium chloride, and add 28% methanolic hydrogenchloride solution (25ml) dropwise to the filtrate at -4°C ) to replace ions, dropwise added ethyl acetate (500ml) to crystallize, kept stirring at -7°C for 1h, and filtered to obtain a crude product.
[0038] Add methanol (100ml) to the crude product to cool down to -7°C, add dropwise 28% methanolic hydrogen chloride solution (6ml), beat for 4 hours, and filter to obtain a beating solid; % hydrogen chloride methanol solution (3ml), beating for 4h, the beat...
Embodiment 1-C
[0039] Example 1-C: Preparation of nicotinamide nucleoside chloride
[0040] A mixture of triacetyl nicotinamide nucleoside trifluoromethanesulfonate (53g, 0.1mol) and methanol (50ml) was added dropwise to 7N ammonia methanol solution (100ml) at -8°C, and the mixed solution was kept at -9°C , stirring and reacting for 24 hours, sampling and testing the remaining 1% of the raw material, the reaction is over, the temperature of the solution is controlled at -7°C, and concentrated sulfuric acid (29g, 0.3mol) is added dropwise to neutralize, the ammonium sulfate is removed by filtration, and the filtrate is controlled at -8°C and 28 % methanolic hydrogen chloride solution (40ml) to replace ions, dropwise added ethyl acetate (600ml) to crystallize, keep stirring at -9°C for 1h, and filter to obtain the crude product.
[0041] Add methanol (100ml) to the crude product and cool down to -8°C, add dropwise 28% hydrogen chloride methanol solution (6ml), beat for 4 hours, and filter to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com