Alcoholic liver disease treating and preventing drug and composition thereof
A technology for alcoholic liver disease and a composition, which is applied in the field of medicine, can solve the problems that the pathological index evaluation system is slightly incomplete, and the therapeutic effect of fat-related diseases and liver diseases is not discussed, and achieves good popularization and application value. Medicinal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
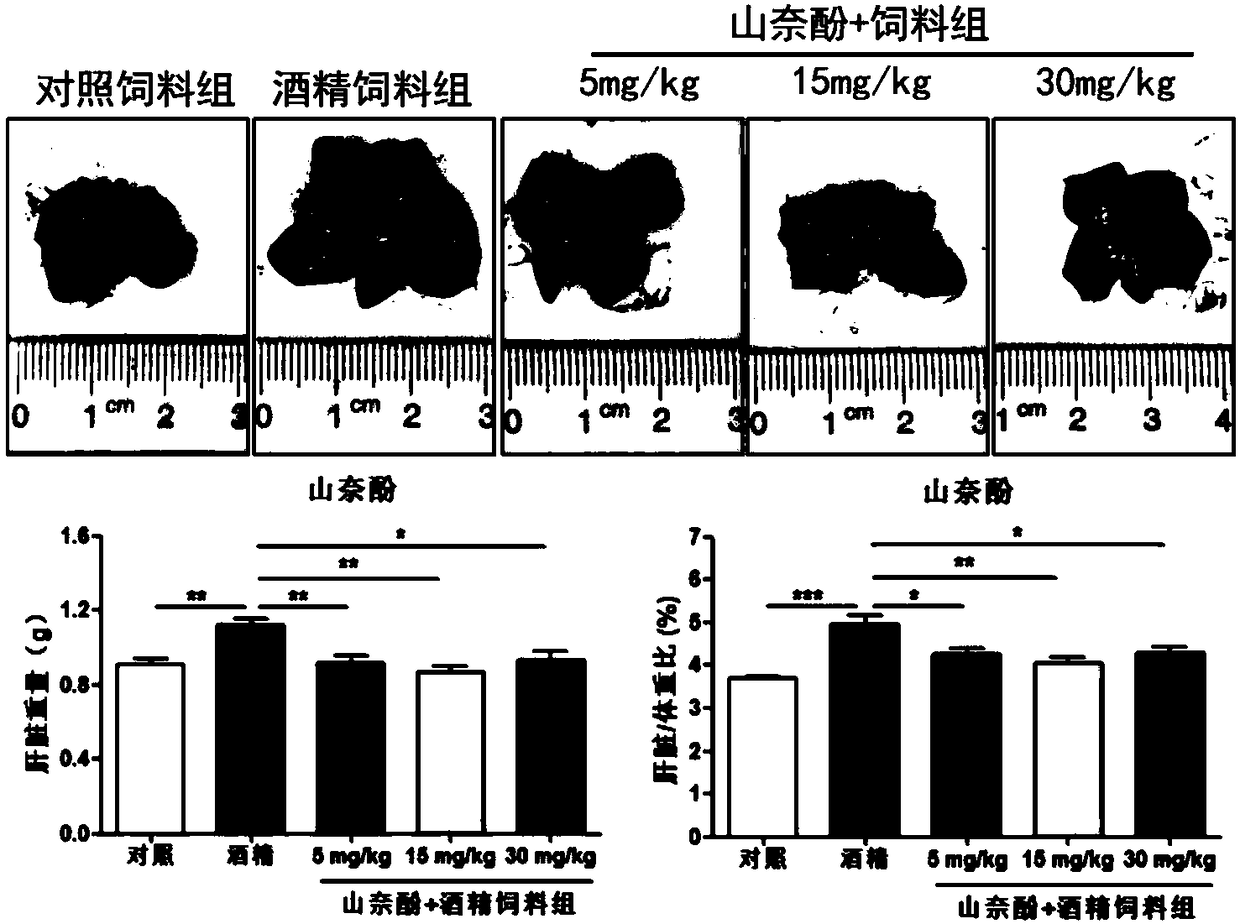
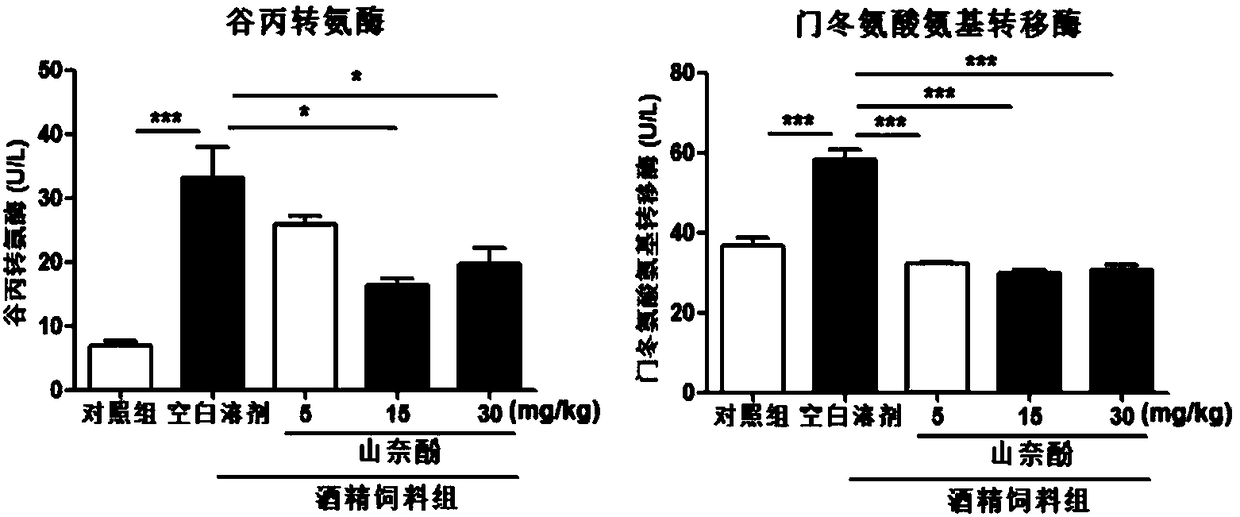
[0063] Different doses of kaempferol were used to prevent and treat alcoholic liver disease caused by NIAAA model.
[0064] 1. Instruments and materials:
[0065] Main instruments: full-wavelength microplate reader (Thermo Company, USA).
[0066] Drugs and reagents: liquid feed (Lieber-DeCarli diet ad libitum) and liquid alcohol feed (Nantong Trophy Feed Technology Co., Ltd.); alcohol (Burdick&Jackson); kaempferol (≥98%, Chengdu Mansite Biotechnology Co., Ltd.) ; ALT, AST, TG detection kits (Nanjing Jiancheng Institute of Bioengineering); other reagents are of analytical grade.
[0067] 2. Experimental animals and dosing regimen:
[0068] C57BL / 6 mice, male, 8-9 weeks old, provided by the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine, were fed with control liquid feed or liquid alcohol feed according to different groups. Mice were fed a control liquid diet (Lieber-DeCarli diet adlibitum) for 5 days; they were acclimated to liquid diet. ...
Embodiment 2
[0088] β-sitosterol is used to prevent and treat alcoholic fatty liver caused by NIAAA model.
[0089] 1. Instruments and materials
[0090] β-sitosterol (≥98%, Chengdu Master Biological Technology Co., Ltd.); the rest are the same as in Example 1.
[0091] 2. Experimental animals and dosing regimen
[0092] The mice were randomly divided into 3 groups, 6 in each group, and the groups were blank control liquid feed group, alcohol modeling group, and alcohol modeling co-administration group. Wherein the alcohol modeling co-administration group contains β-sitosterol (10 / 20 / 30mg / kg); the rest are the same as in Example 1.
[0093] Liver tissue and serum chemical detection, mathematical statistics are the same as in Example 1.
[0094] 3. Experimental results
[0095] General situation: Compared with the control liquid feed group, the liver volume, rough surface and color change of the alcohol modeling group can be clearly observed by the naked eye after dissection. After syn...
Embodiment 3
[0109] Tanshinone I is used to prevent and treat alcoholic fatty liver caused by NIAAA model.
[0110] 1. Instruments and materials
[0111] Tanshinone I (≥98%, Chengdu Master Biological Technology Co., Ltd.); the rest are the same as in Example 1.
[0112] 2. Experimental animals and dosing regimen
[0113] Mice are divided into 5 groups at random, 6 in every group, and group is blank control liquid feed group, alcohol modeling group, alcohol modeling cooperating administration group: wherein the dosage of Tanshinone I is: 15mg / kg, 30mg / kg, 60mg / kg, all the other are with embodiment 1.
[0114] Liver tissue and serum chemical detection, data statistics are the same as in Example 1.
[0115] Experimental results:
[0116] Compared with the control liquid feed group, the larger liver volume, rough surface, and color change of the alcohol modeling group could be clearly observed by the naked eye after dissection. After co-administration of the above doses, the above patholo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com