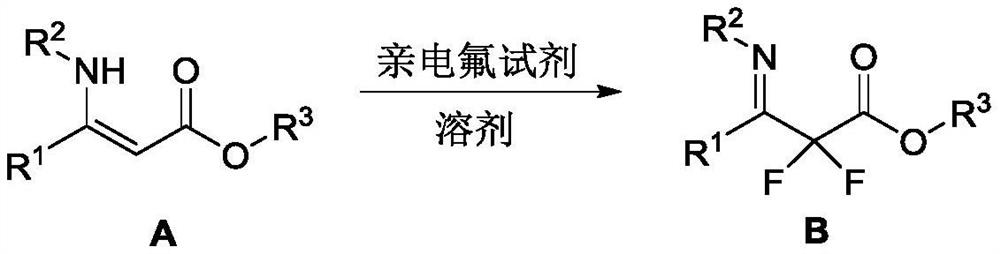
A kind of imine compound substituted by difluoroacetate and preparation method thereof
A technology of imine compound and difluoroacetate, which is applied in the preparation of imino compound and organic chemistry, etc., can solve the problem of strong electron-absorbing ability of imine unstable difluoromethylene, difficulty in conversion of functional groups of reaction products, reaction Difficult preparation of substrates and other problems, to achieve the effect of wide application range of substrates, overcoming the limitations of reaction substrates, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
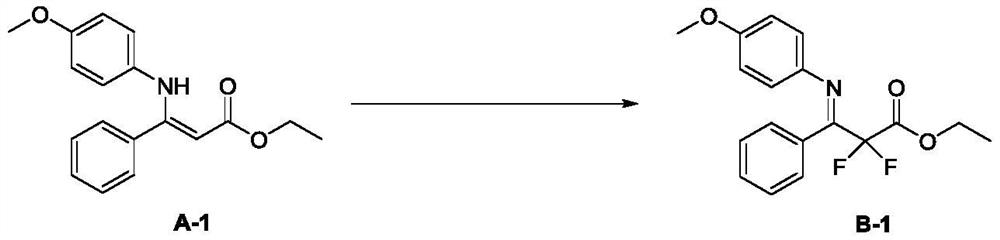
[0019] In a dry 25 mL reaction flask, A-1 (179 mg, 0.6 mmol) was dissolved in acetonitrile (6 mL). After the system was cooled to 0 °C, Selectfluor (532 mg, 1.5 mmol) was added at one time, and continued at 0 °C. The reaction was stirred for 1 h. After TLC detected that the reaction was complete, diethyl ether (5 mL) was added to the system, then the insolubles were removed by filtration, the filter cake was washed three times with diethyl ether (5 mL), and the filtrate was spin-dried by column chromatography (eluent: petroleum ether / ethyl acetate) = 20 / 1 to 10 / 1) to obtain the target product (190 mg, yield 95%) as a bright yellow oily liquid. 1 H NMR (500MHz, CDCl 3 )δ(ppm): 7.39–7.32 (m, 3H), 7.28–7.26 (m, 2H), 6.69 (s, 4H), 4.44 (q, J=7Hz, 2H), 3.73 (s, 3H), 1.40 (t, J=7Hz, 3H); 13 C NMR (125MHz, CDCl 3 )δ(ppm): 163.36(t, J=31Hz), 159.81(t, J=30Hz), 157.86, 140.02, 131.00, 130.04, 128.91, 128.72, 123.64, 114.01, 113.13(t, J=253Hz), 63.07 ,55.45,14.19; 19 F...
Embodiment 2
[0021]
[0022] In a dry 25 mL reaction flask, A-1 (179 mg, 0.6 mmol) was dissolved in acetonitrile (6 mL). After the system was cooled to 0 °C, Accufluor (483 mg, 1.5 mmol) was added at one time, and stirring was continued at room temperature. The reaction was carried out for 2h. After TLC detected that the reaction was complete, diethyl ether (5 mL) was added to the system, then the insolubles were removed by filtration, the filter cake was washed three times with diethyl ether (5 mL), and the filtrate was spin-dried by column chromatography (eluent: petroleum ether / ethyl acetate) = 20 / 1 to 10 / 1) to obtain the target product (106 mg, yield 53%) as a bright yellow oily liquid. 1 H NMR (500MHz, CDCl 3 )δ(ppm): 7.39–7.32 (m, 3H), 7.28–7.26 (m, 2H), 6.69 (s, 4H), 4.44 (q, J=7Hz, 2H), 3.73 (s, 3H), 1.40 (t, J=7Hz, 3H); 13 C NMR (125MHz, CDCl 3 )δ(ppm): 163.36(t, J=31Hz), 159.81(t, J=30Hz), 157.86, 140.02, 131.00, 130.04, 128.91, 128.72, 123.64, 114.01, 113.13(t, J=253Hz), ...
Embodiment 3
[0024]
[0025] In a dry 25mL reaction flask, A-1 (179mg, 0.6mmol) was dissolved in 1,4-dioxane (6mL), Selectfluor (532mg, 1.5mmol) was added at one time at room temperature, and the reaction was continued with stirring 1h. After TLC detected that the reaction was complete, diethyl ether (5 mL) was added to the system, then the insolubles were removed by filtration, the filter cake was washed three times with diethyl ether (5 mL), and the filtrate was spin-dried by column chromatography (eluent: petroleum ether / ethyl acetate) = 20 / 1 to 10 / 1) to obtain the target product (137 mg, yield 69%) as a bright yellow oily liquid. 1 H NMR (500MHz, CDCl 3 )δ(ppm): 7.39–7.32 (m, 3H), 7.28–7.26 (m, 2H), 6.69 (s, 4H), 4.44 (q, J=7Hz, 2H), 3.73 (s, 3H), 1.40 (t, J=7Hz, 3H); 13 CNMR (125MHz, CDCl 3 )δ(ppm): 163.36(t, J=31Hz), 159.81(t, J=30Hz), 157.86, 140.02, 131.00, 130.04, 128.91, 128.72, 123.64, 114.01, 113.13(t, J=253Hz), 63.07 ,55.45,14.19; 19 F NMR (376MHz, CDCl 3 )δ(ppm):–10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com