Fluorescent selective histone deacetylase inhibitor and preparation method and application thereof
A deacetylase and histone technology, applied in chemical instruments and methods, medical preparations containing active ingredients, organic chemistry, etc., can solve the problem of lack of HDAC6 subtype selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Synthesis of Compounds 6a and 6b
[0044] synthetic route:
[0045]
[0046] Reagents and conditions: a) triethylamine, ethanol, microwave heating at 100°C; b) morpholine, 5-bisdiphenylphosphine-9,9-dimethylxanthene, bis(dibenzylideneacetone)palladium , cesium carbonate, toluene, 65°C; c) lithium hydroxide, water / THF; d) oxalyl chloride, N,N-dimethylformamide, dichloromethane, then hydroxylamine hydrochloride, triethylamine, water / THF.
[0047] Concrete synthesis method and steps are as follows:
[0048] Intermediate 3a: Methyl 4-((6-bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl)benzoate
[0049] Starting materials 6-Bromo-1H,3H-benzo[de]isochromene-1,3-dione (1,1.35g, 4.87mmol), methyl 4-(aminomethyl)benzoate Acid salt (2a, 4-982mg, 4.87mmol) and triethylamine (493mg, 4.87mmol) were dissolved in 20mL of ethanol, heated to 100°C for 45 minutes by microwave. The reaction solution was diluted with 100 mL of water, and extracted three times with...
Embodiment 2
[0064] Example 2. In vitro HDACs inhibitory activity evaluation experiment of target compounds
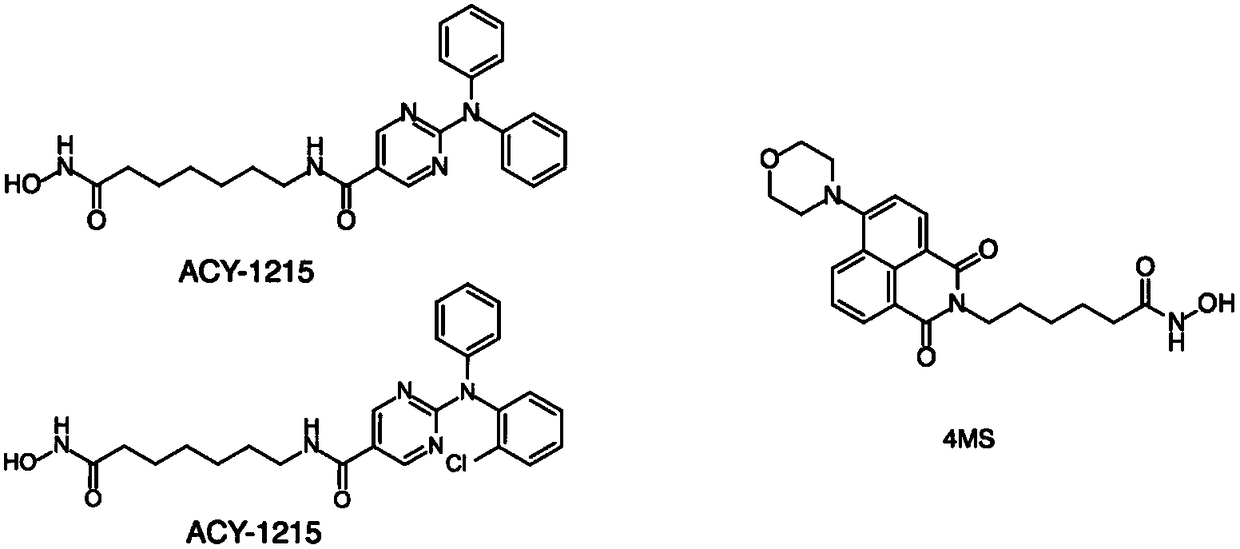
[0065] References [Duan, W.; Li, J.; Inks, E.S.; Chou, C.J.; Jia, Y.; Chu, X.; Li, X.; Xu, W.*; Zhang, Y.* Design, Synthesis and Antitumor Evaluation of Novel HistoneDeacetylase (HDAC) Inhibitors Equipped with Phenylsulfonylfuroxan Module as Nitric Oxide (NO) Donor. J. Med. Chem. 2015, 58 (10), 4325-4338.] Related methods to test the reported HDACs inhibition In vitro HDACs inhibitory activity of agent 4MS and compounds 6a and 6b of the present invention.
[0066] The test results (Table 1) show that the compound 6a of the present invention and the reported HDACs inhibitor 4MS have strong inhibitory activity on HDAC1, HDAC2, HDAC3 of class I subfamily and HDAC6 of class IIb subfamily, without subtype Selectivity; while compound 6b of the present invention shows strong subtype selectivity to HDAC6 of class IIb subfamily.
[0067] Table 1. Evaluation results of HDACs inhibitory acti...
Embodiment 3
[0070] Example 3. Target compound protein immunoblotting (Western blot) evaluation experiment
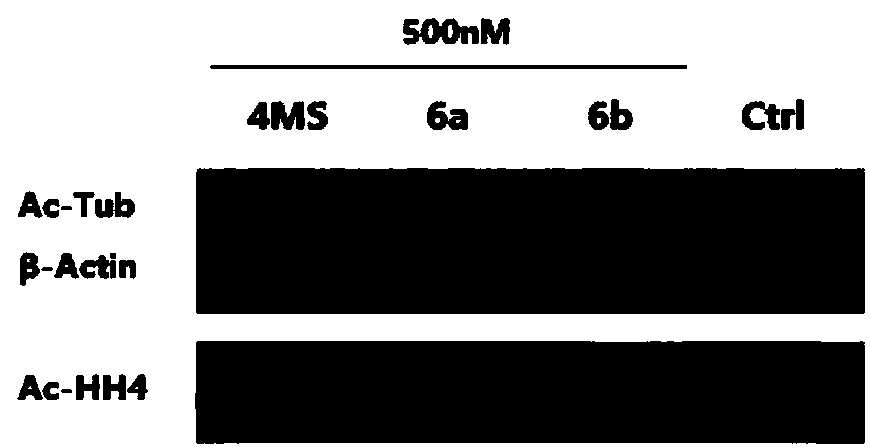
[0071] The HDACs inhibitory activity of the reported HDACs inhibitor 4MS and compounds 6a and 6b of the present invention in cells was evaluated by Western blot test.
[0072] Test principle: HDAC1, HDAC2 and HDAC3 of class I subfamily can deacetylate acetylated histone H4 (Ac-HH4), thereby reducing the protein level of Ac-HH4 in cells; HDAC6 of class IIb subfamily can deacetylate Tubulin (Ac-Tub) is deacetylated, thereby reducing the protein level of Ac-Tub in cells. Therefore, the inhibitory effect of the compound on intracellular HDACs can be evaluated by measuring the Ac-HH4 and Ac-Tub protein levels in the cells treated with the compound by Western blot assay.
[0073] Experimental materials and methods: Human non-small cell lung cancer A549 cells were treated with 4MS, 6a and 6b at a final concentration of 500nM respectively, and the cells were collected. The cells were lysed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com