Method for restoring antibacterial activity of tigecycline
A technology of tigecycline and antibacterial activity, applied in the field of biology and medicine, can solve the problems of false drug resistance and achieve the effect of restoring sensitivity, accurate results and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Disk method drug susceptibility test
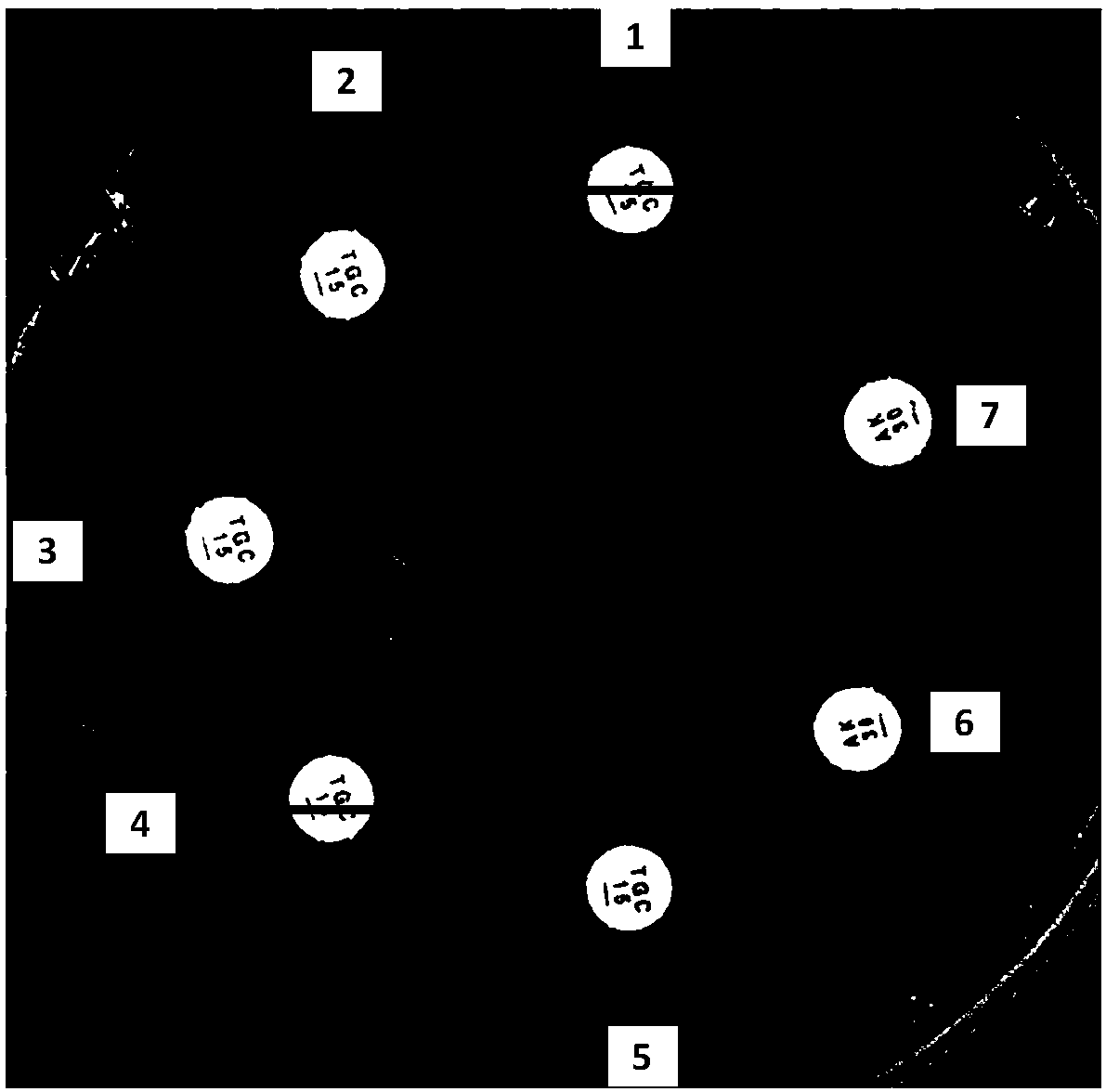
[0037] According to the requirements of the American Association for Clinical and Laboratory Standardization, the standard disc diffusion method drug susceptibility test was carried out, and a suspension of Klebsiella pneumoniae 0.5 turbidites was evenly spread on the Mueller-Hinton agar plate, and dried for 15 minutes. Then paste the tigecycline and amikacin drug sensitive paper respectively, and measure the diameter of the inhibition zone after overnight incubation. After adding different concentrations of resensitization solution (the resensitization solution is EDTA solution, the concentration range is 100-400μg / sheet) (No. 2-5 paper), the inhibition zone expands, such as the inhibition zone of No. 4 tigecycline is 22mm; in order to prove that the resensitization solution itself has no inhibitory effect on bacteria, two amikacin paper sheets (No. 6 and No. 7) were pasted in this experiment. No. 6 paper was added with...
Embodiment 2
[0038] Example 2: Drug susceptibility test by agar dilution method
[0039] According to the requirements of the American Association for Clinical and Laboratory Standardization, the standard agar dilution method drug susceptibility test was carried out. The suspension of 18 strains of Klebsiella pneumoniae 0.5 M. turbidis was diluted 10 times and then inoculated in a series of with and without resensitization solution. On the MHA agar plate of tigecycline concentration, the drug susceptibility test results showed that in the presence of the resensitization solution, 9 of the 18 bacteria recovered their sensitivity to tigecycline, and their MIC values ranged from > 8 μg / g / mL down to ≤1 μg / mL (eg figure 2 shown).
Embodiment 3
[0040] Example 3: Micro-broth dilution method drug susceptibility test
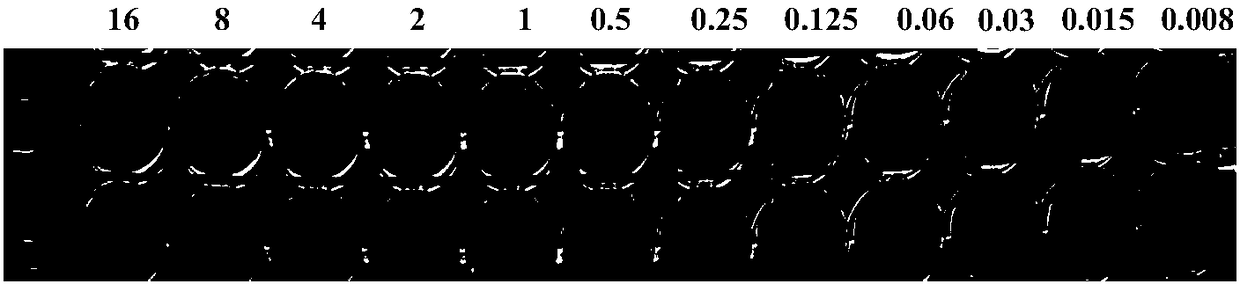
[0041] According to the requirements of the American Association for Clinical and Laboratory Standardization, the standard micro-broth dilution method drug susceptibility test was carried out, and a Klebsiella pneumoniae 0.5 turbidity bacteria suspension was diluted 100 times and then inoculated with and without resensitization solution In a series of tigecycline concentration microplates, the drug susceptibility test results showed that in the presence of resensitization solution, the bacteria recovered their sensitivity to tigecycline, and the MIC value decreased from >16 μg / mL to 1 μg / mL. mL (e.g. image 3 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com