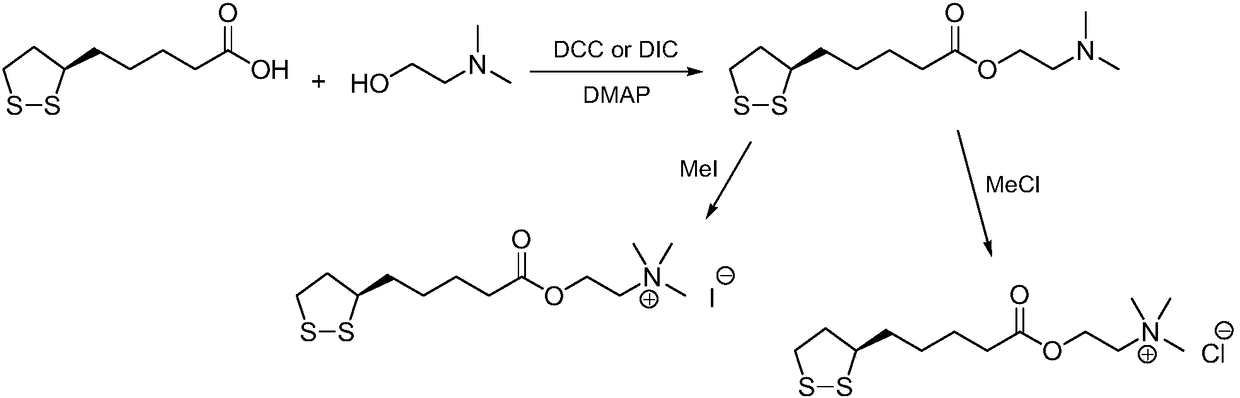
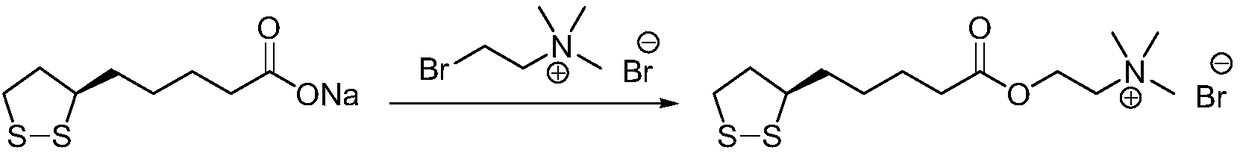
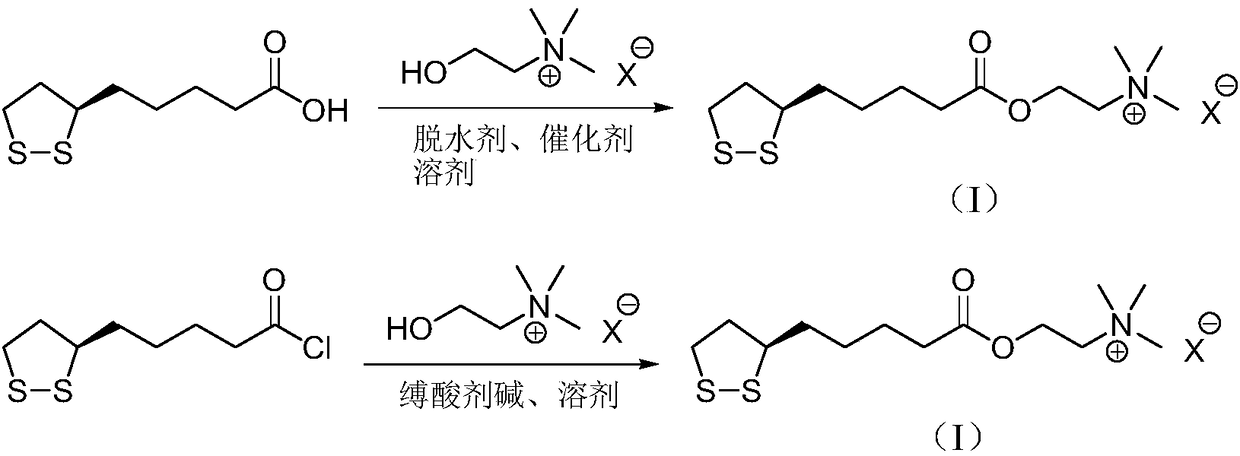
Refining method of R-thioctic choline ester halide
A kind of technology of lipoic acid choline and refining method, applied in the refining field of R-lipoic acid choline ester halide, can solve the problems such as not giving R-lipoic acid choline ester halide, etc., to improve purity and quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add R-lipoic acid (10.0g) and chloroform (120mL) into the reaction flask, stir to dissolve, add N,N'-dicyclohexylcarbodiimide (12.0g) and 4-dimethylaminopyridine (1.2g) , cooled in an ice bath, choline chloride (15.2 g) was added, and the reaction mixture was reacted at 35° C. for 18 h. Filtrate, extract the filter cake with ethyl acetate, combine the extracts and filtrate, and rotary evaporate to dryness under reduced pressure. The residue (31g) was dissolved in chloroform (80mL), cooled to room temperature, added silica gel (60g), and stirred at 35°C until uniform. Filtration, the filtrate was rotary evaporated to 30mL under reduced pressure, cooled to 0°C and crystallized for 12h, filtered, and dried to obtain R-lipoic acid choline ester chloride, a light yellow solid (14.1g), yield 89%, liquid phase detection, R-lipoic acid choline ester chloride is greater than 99.7%, and the raw material content of choline chloride is less than 0.1%.
Embodiment 2
[0025] Add R-lipoyl chloride (10.0g) and toluene (160mL) into the reaction flask, stir to dissolve, add N,N-diethylaniline (26.6g), cool in an ice bath, add choline chloride (18.6g), The reaction mixture was reacted at 20°C for 12h. Filtrate, extract the filter cake with ethyl acetate, combine the extracts with the filtrate, and rotary evaporate to dryness under reduced pressure. The residue (30g) is dissolved in toluene (100mL), cooled to room temperature, added with silica gel (85g), and stirred at 40°C. Filtration, the filtrate was rotary evaporated to 30mL under reduced pressure, cooled to 1°C and crystallized for 16h, filtered, and dried to obtain R-lipoic acid choline ester chloride, a light yellow solid (11.3g), yield 78%, liquid phase detection, R-lipoic acid choline ester chloride is greater than 99.7%, and the raw material content of choline chloride is less than 0.1%.
Embodiment 3
[0027] Add R-lipoic acid (10.0g) and ethyl acetate (150mL) into the reaction flask, stir to dissolve, add N,N'-carbonyldiimidazole (8.6g) and 2,6-lutidine (0.5g), After cooling in an ice bath, choline bromide (26.8 g) was added, and the reaction mixture was reacted at 20° C. for 24 h. Filtrate, extract the filter cake with ethyl acetate, combine the extracts and filtrate, and rotary evaporate to dryness under reduced pressure. The residue (29g) was dissolved in ethyl acetate (77mL), cooled to room temperature, added silica gel (32g), and stirred at 32°C Uniform, filtered, and the filtrate was rotary evaporated to 30mL under reduced pressure, cooled to 2°C and crystallized for 14h, filtered, and dried to obtain R-lipoic acid choline ester bromide, light yellow solid (15.4g), yield 85%, R- The lipoic acid choline ester bromide is greater than 99.7%, and the raw material content of choline bromide is less than 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com