Gas diffusion electrode, its preparation method and its application in the electrochemical reduction of carbon dioxide
A gas diffusion electrode and carbon dioxide technology, applied in the field of electrochemical catalysis, can solve the problems of low energy utilization rate of electrochemical reduction of carbon dioxide, easy deactivation of catalyst, poor product selectivity, etc., to increase the catalytic activity of electrochemical reduction and inhibit hydrogen evolution Reaction, the effect of increasing the contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: catalyst preparation
[0045] Weigh 10g of urea and 1g of anhydrous copper acetate, mix urea and anhydrous copper acetate evenly and physically to obtain a catalyst precursor, place the catalyst precursor in a 30mL crucible with a lid, and place it in a muffle furnace for pyrolysis Reaction, the reaction temperature is 400°C, the heating rate is 5°C / min, and the reaction time is 2h. The obtained solid is ground to obtain graphite-phase carbon nitride-supported nano-copper oxide, which is the carbon dioxide electrochemical reduction catalyst, called Cu x O@C 3 N 4 -400°C catalyst.
Embodiment 2
[0046] Embodiment 2: catalyst preparation
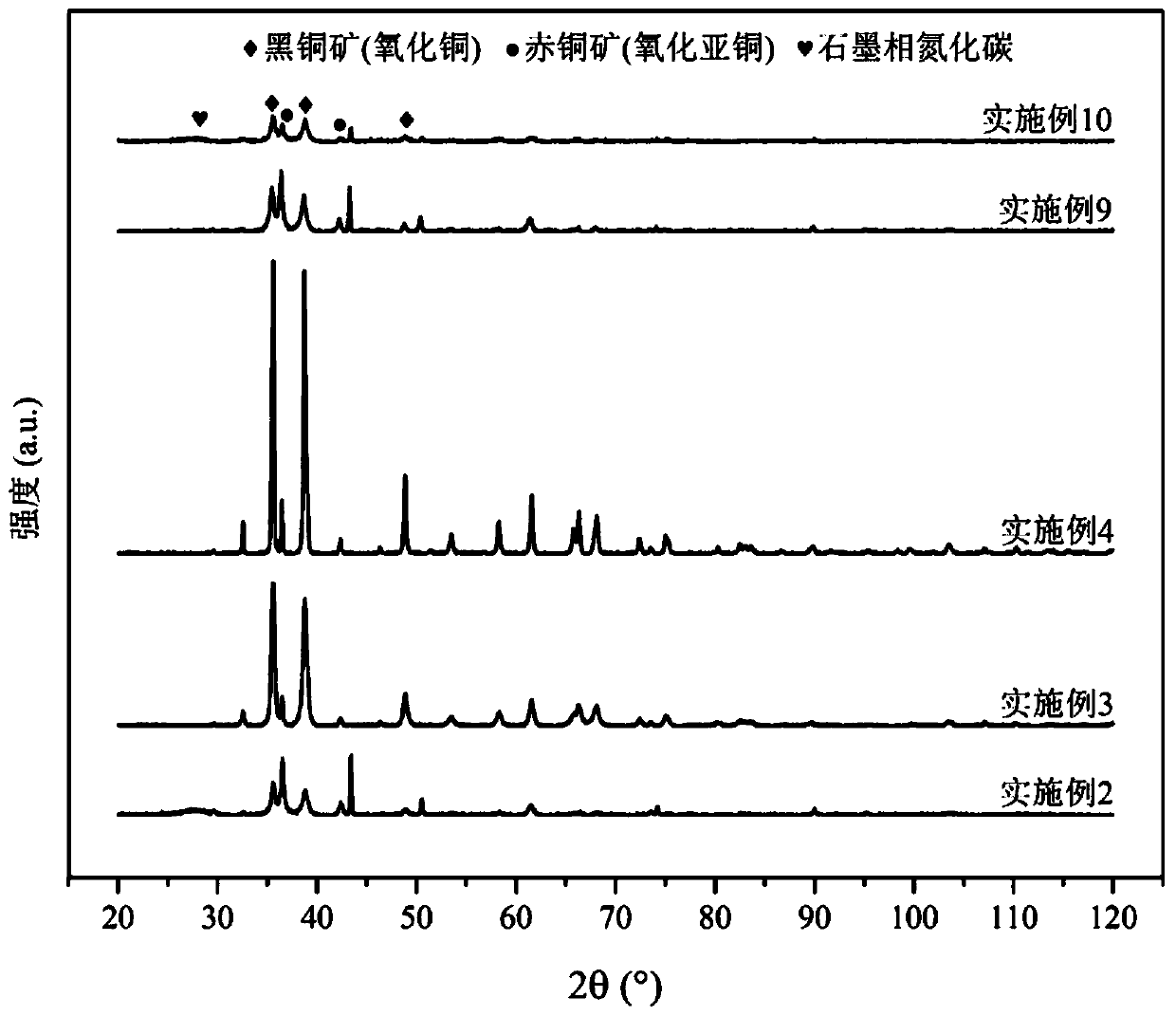
[0047] The specific preparation process is as shown in Example 1. Only by changing the reaction temperature to 450°C, the graphite-phase carbon nitride-supported nano-copper oxide can also be obtained, which is called Cu x O@C 3 N 4 -450°C catalyst. For Cu in Example 2 x O@C 3 N 4 XRD characterization of the catalyst at -450°C, such as figure 1 As shown, it can be seen that the catalyst includes graphite phase carbon nitride and nano-copper oxides in two crystal forms of cupolite and cuprite.
Embodiment 3
[0048] Embodiment 3: catalyst preparation
[0049] The specific preparation process is as shown in Example 1. Only by changing the reaction temperature to 500 ° C, can also obtain graphite-phase carbon nitride-supported nano-copper oxide, called Cu x O@C 3 N 4 -500°C catalyst. For Cu in Example 3 x O@C 3 N 4 XRD characterization of the catalyst at -500°C, such as figure 1 As shown, it can be seen that the catalyst includes graphite phase carbon nitride and nano-copper oxides in two crystal forms of cupolite and cuprite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com