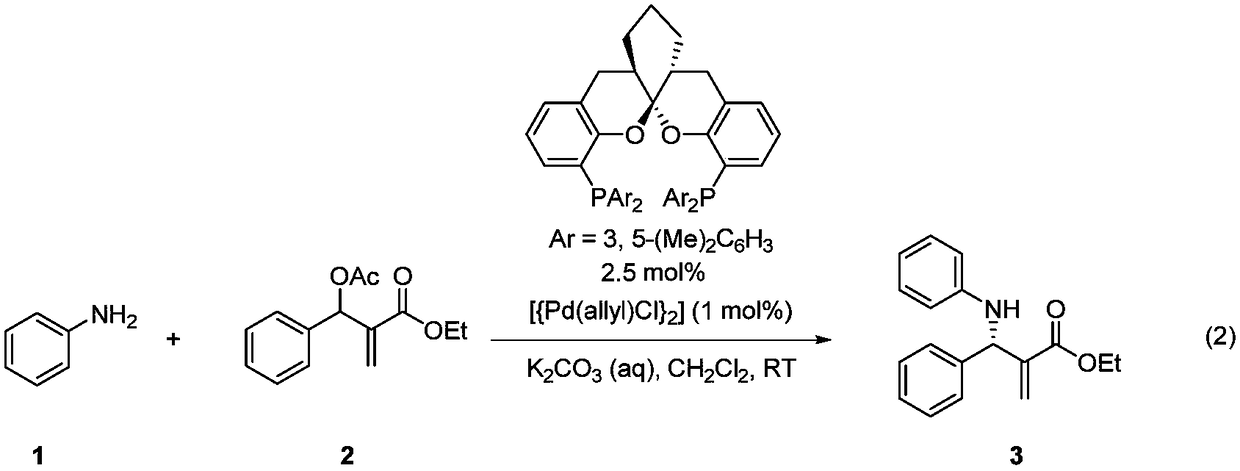
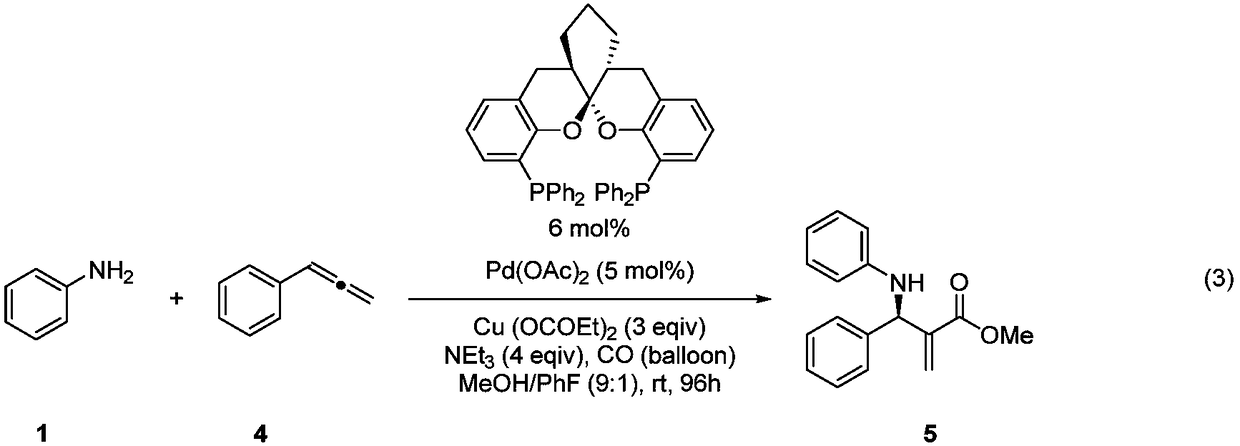
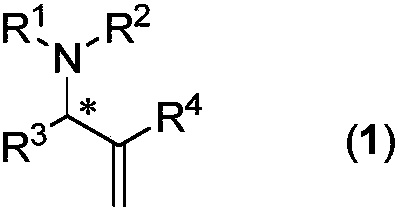
Chiral aryl allyl amine compound and synthesis method thereof
A technology of aryl allyl amine and amine compounds, which is applied in the field of chiral aryl allyl amine compounds and their synthesis, can solve the problems of complex chiral ligand structures, residual metal products, and expensive catalysts, etc. Achieve the effect of low cost, high optical purity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] At room temperature, dissolve 9.3mg of aniline and 14.6mg of MBH adduct with tert-butoxyloxyl group in 0.5mL of p-xylene, add 17.1mg of hydroquinidine (anthraquinone-1,4-diyl) Diether ((DHQD) 2AQN) and 19.5mg of calcium fluoride were stirred at room temperature for 72 hours, the solvent was removed, and the residue was separated by column chromatography to obtain the desired compound, whose molecular structural formula was: It is a colorless oil, 80% yield, 92% ee, [determined by high performance liquid chromatography, chiral OD-H column, n-hexane:isopropanol=95:5, 0.5mL / min, 254nm, t R (major)=8.57min,t R (minor)=10.76min]. Optical rotation value (c=0.7, CHCl 3 ), 1 H NMR (300MHz, CDCl 3 ):δ=7.28-7.40(m,5H),7.14-7.20(m,2H),6.70-6.74(m,1H),6.58(dd,J=8.6Hz,J=0.9Hz,2H),6.40( s,1H),5.97(t,J=1.2Hz,1H),5.41(s,1H),4.16(s,1H),3.70(s,3H); 13 C NMR (100MHz, CDCl 3 ):166.8,146.8,140.7,140.0,129.3,128.9,128.0,127.7,126.3,118.0,113.5,59.0,52.0.HRMS(ESI):C 17 h 18 NO 2 ...
Embodiment 2
[0036] At room temperature, 19.7 mg of 1,8-naphthalimide and 14.6 mg of MBH adducts with tert-butoxyacyloxy were dissolved in 0.5 mL of p-xylene, and 17.1 mg of hydrogenated quinidine (anthracene Quinone-1,4-diyl)diether ((DHQD)2AQN) and 19.5mg of calcium fluoride were stirred at room temperature for 72 hours, the solvent was removed, and the residue was separated by column chromatography to obtain the desired compound, which The molecular structural formula is: It is a colorless oil, 71% yield, 92% ee, [determined by high performance liquid chromatography, chiral AD-H column, n-hexane:isopropanol=90:10, 1mL / min, 254nm, t R(major)=24.84min,t R (minor) = 29.00 min]. Optical rotation value (c=0.3, CH 2 Cl 2 ), 1 H NMR (300MHz, CDCl 3 ): δ=8.56(d, J=7.2Hz, 2H), 8.16(d, J=8.2Hz, 2H), 7.70(t, J=7.8Hz, 2H), 7.57(d, J=7.4Hz, 2H ),7.26-7.36(m,3H),7.20(s,1H),6.47(d,J=1.4Hz,1H),5.60(d,J=1.7Hz,1H),3.67(s,3H); 13 C NMR (100MHz, CDCl 3 ):166.7,164.4,139.2,137.8,134.0,131.6,131....
Embodiment 3
[0038] At room temperature, 12.5 mg of methyl 2-pyrrolecarboxylate and 14.6 mg of MBH adduct with tert-butoxyloxyl group were dissolved in 0.5 mL of p-xylene, and 17.1 mg of hydroquinidine (anthraquinone-1, 4-diyl) diether ((DHQD) 2AQN) and 19.5mg of calcium fluoride were stirred at 50°C for 72 hours, the solvent was removed, and the residue was separated by column chromatography to obtain the desired compound, whose molecular structure was : It is a colorless oil, 50% yield, 84% ee, [determined by high performance liquid chromatography, chiral AD-H column, n-hexane:isopropanol=90:10, 1mL / min, 254nm, t R (major)=7.99min,t R (minor)=9.92min]. Optical rotation value (c=0.44, CH 2 Cl 2 ), 1 H NMR (300MHz, CDCl 3 ):δ=7.49(s,1H),7.31-7.37(m,3H),7.21(d,J=7.4Hz,2H),7.04(t,J=1.9Hz,1H),6.63(s,1H) ,6.44(s,1H),6.11(t,J=3.1Hz,1H),5.01(s,1H),3.78(s,3H),3.70(s,3H); 13 C NMR (100MHz, CDCl 3 ):165.9,161.4,141.4,137.78,128.9,128.6,128.4,127.5,127.3,122.2,119.1,108.3,61.2,52.4,51.3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com