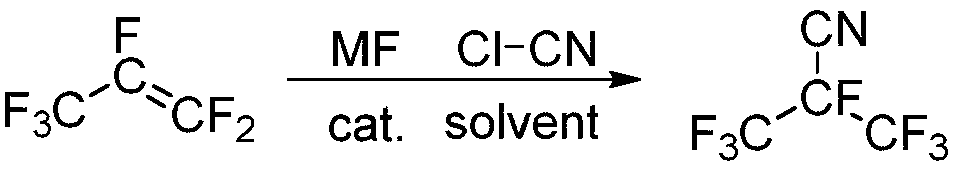Preparation method of perfluoronitrile type compound
A compound and perfluoronitrile technology, applied in the field of preparation of perfluoronitrile compounds, can solve the problems of low yield and uneconomical perfluoronitrile compounds, and achieve the effects of easy availability of raw materials, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Under the protection of nitrogen replacement, 12.76g (0.22mol) of potassium fluoride, 12.29g (0.20mol) of cyanogen chloride, and 100mL of acetonitrile were added to a 500mL dry autoclave, and 33.00g ( 0.22mol), 50 ℃ airtight reaction for 10 hours. After the reaction is completed, the reaction solution is volatilized at 5°C in an alcohol low-temperature cooling tank, and the volatile components are collected at -40°C. The product perfluoroisobutyronitrile at -5°C was 27.60 g, and the yield was 70.4%.
Embodiment 2
[0040] Under the protection of nitrogen replacement, 9.24g (0.22mol) of sodium fluoride, 12.29g (0.20mol) of cyanogen chloride, and 100mL of acetonitrile were added to a 500mL dry autoclave, and 33.00g ( 0.22mol), 50 ℃ airtight reaction for 10 hours. After the reaction is completed, the reaction liquid is volatilized at 5°C in an alcohol low-temperature cooling tank, and the volatile components are collected at -40°C, and the volatile components are volatilized at -20°C to remove unreacted hexafluoropropylene, and then volatilized and collected to obtain the boiling point It was 27.51 g of perfluoroisobutyronitrile at -5°C, and the yield was 70.2%.
Embodiment 3
[0042] Under the protection of nitrogen replacement, add 12.76g (0.22mol) of potassium fluoride (0.22mol), 12.29g (0.20mol) of cyanogen chloride, and 100mL of diethylene glycol dimethyl ether into a 500mL dry autoclave. 33.00 g (0.22 mol) of perfluoropropene was reacted at 50° C. for 10 hours in a sealed manner. After the reaction is completed, the reaction solution is volatilized at 5°C in an alcohol low-temperature cooling tank, and the volatile components are collected at -40°C, and the volatile components are volatilized at -20°C to remove unreacted hexafluoropropylene, and then evaporated and collected to obtain the boiling point It was 25.60 g of perfluoroisobutyronitrile at -5°C, and the yield was 65.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com