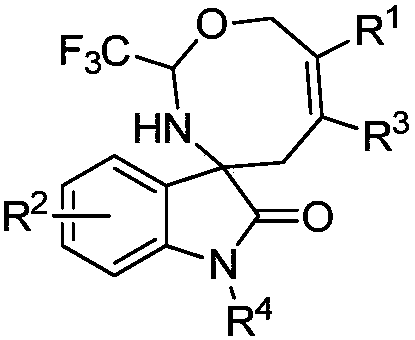
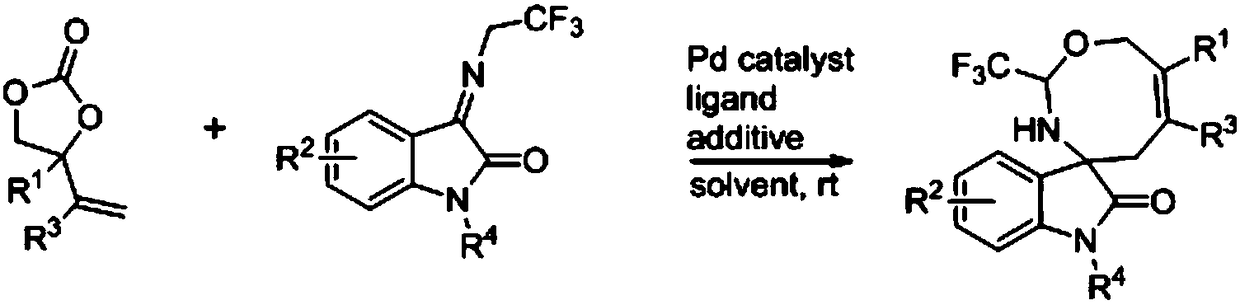
Eight-membered nitrogen-oxygen heterocyclic spiro indolone compound and preparation method thereof
A technology for heterocyclic spiro indolones and compounds is applied in the field of compound preparation, can solve rare problems and the like, and achieves the effects of short time, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
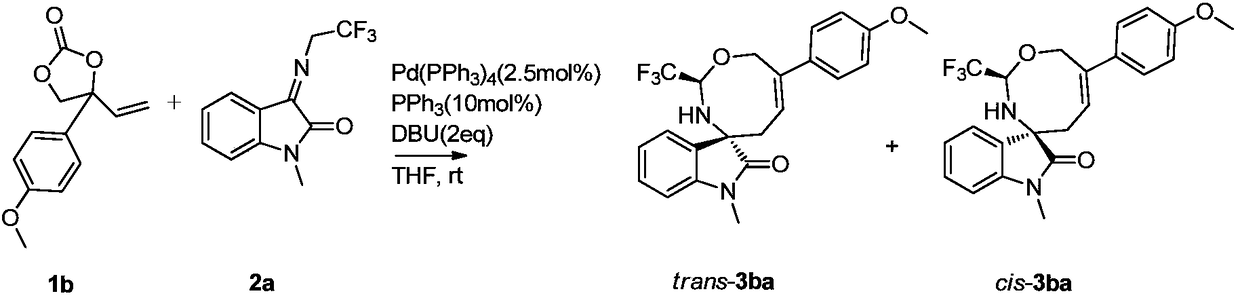
[0037] Weigh 1b (22.0mg, 0.1mmol), 2a (24.2mg, 0.1mmol), palladium catalyst (2.89mg, 0.0025mmol) and ligand PPh 3 (2.6mg, 0.01mmol) was dissolved in 1mL of tetrahydrofuran, added DBU (29.8μL, 0.2mmol) and stirred for 2 hours (detect the reaction with TLC), after the reaction was complete, the crude product was subjected to column chromatography (the eluent was selected as volume ratio of 8:1 petroleum ether / ethyl acetate mixed solution) to obtain the target product trans-3ba (11.7mg), with a yield of 28%; cis-3ba (9.6mg), with a yield of 23%.
[0038] Characterization and analysis of target objects:
[0039] trans-3ba: yellow solid, 1 H NMR (400MHz, CDCl 3 ):δ7.48-7.44(m,2H),7.34(td,J=7.8,1Hz,1H),7.18(d,J=7.2Hz,1H),7.04(t,J=7.48Hz,1H), 6.94-6.90(m, 2H), 6.87(d, J=7.8Hz, 1H), 6.23(t, J=8.5Hz, 1H), 5.70-5.64(m, 1H), 4.76(d, J=13.6Hz ,1H),4.63(d,J=13.6Hz,1H),3.84(s,3H),3.22(s,3H),3.02(s,1H),2.53(s,1H),2.45(d,J= 10.2Hz,1H)ppm; HRMS(ESI)m / z:C 22 h 21 f 3 N 2 o ...
Embodiment 2
[0042]
[0043]Weigh 1c (26.8mg, 0.1mmol), 2c (24.2mg, 0.1mmol), palladium catalyst (2.89mg, 0.0025mmol) and ligand PPh 3 (2.6mg, 0.01mmol) was dissolved in 1mL of tetrahydrofuran, added DBU (29.8μL, 0.2mmol) and stirred for 2 hours (detect the reaction with TLC), after the reaction was complete, the crude product was subjected to column chromatography (the eluent was selected as volume The ratio of 8:1 petroleum ether / ethyl acetate mixed solution) can obtain the target product trans-3ca (11.3mg), the yield is 24%; cis-3ca (11.3mg), the yield is 24%.
[0044] Characterization and analysis of target objects:
[0045] trans-3ca: white solid, 1 H NMR (400MHz, CDCl 3 ):δ7.53-7.49(m,2H),7.40-7.37(m,2H),7.34(dd,J=7.7,1.2Hz,1H),7.15(d,J=7.2Hz,1H),7.05( t,J=7.2Hz,1H),6.88(d,J=7.8Hz,1H),6.32(t,J=8.5Hz,1H),5.68-5.62(m,1H),4.76(d,J=13.5 Hz, 1H), 4.60(d, J=13.5, 1H), 3.22(s, 3H), 2.97(s, 1H), 2.57(s, 1H), 2.44(d, J=10.2Hz, 1H)ppm; HRMS(ESI)m / z:C 21 h 18 BrF 3 N 2 o 2 [M+H] +...
Embodiment 3
[0048]
[0049] Weigh 1d (22.4mg, 0.1mmol), 2d (24.2mg, 0.1mmol), palladium catalyst (2.89mg, 0.0025mmol) and ligand PPh 3 (2.6mg, 0.01mmol) was dissolved in 1mL of tetrahydrofuran, added DBU (29.8μL, 0.2mmol) and stirred for 2 hours (detect the reaction with TLC), after the reaction was complete, the crude product was subjected to column chromatography (the eluent was selected as volume The ratio of 8:1 petroleum ether / ethyl acetate mixed solution) can obtain the target product trans-3da (14.5mg), the yield is 34%; cis-3da (13.6mg), the yield is 32%.
[0050] Characterization and analysis of target objects:
[0051] trans-3da: yellow solid, 1 H NMR (400MHz, CDCl 3 ):δ7.47-7.43(m,2H),7.37-7.33(m,3H),7.15(d,J=7.2Hz,1H),7.05(t,J=7.2Hz,1H),6.88(d, J=7.8Hz, 1H), 6.31(t, J=8.5Hz, 1H), 5.68-5.62(m, 1H), 4.77(d, J=13.5Hz, 1H), 4.61(d, J=13.5Hz, 1H), 3.22(s, 3H), 2.98(s, 1H), 2.58(s, 1H), 2.45(d, J=10.2Hz, 1H) ppm; HRMS(ESI) m / z: C 21 h 18 CIF 3 N 2 o 2 [M+H] + The theore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com