Method for recovering copper salt and ethanol from D,L-threo-p-methylsulfonylphenyl serine ethyl ester stock solution
A technology of thiamphenyl phenylserine ethyl ester mother liquor and thiamphenyl phenylserine ethyl ester, which is applied in the fields of medicine and chemical industry, can solve problems such as decline, and achieve the effects of reducing COD, reducing wastewater treatment costs, and reducing product costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
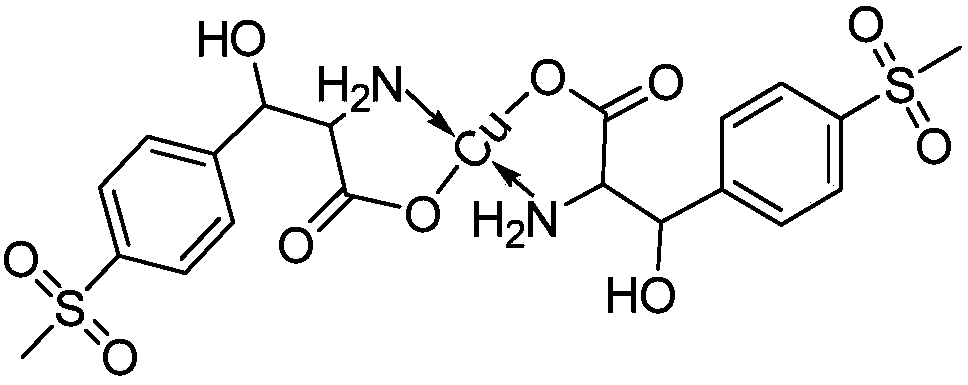
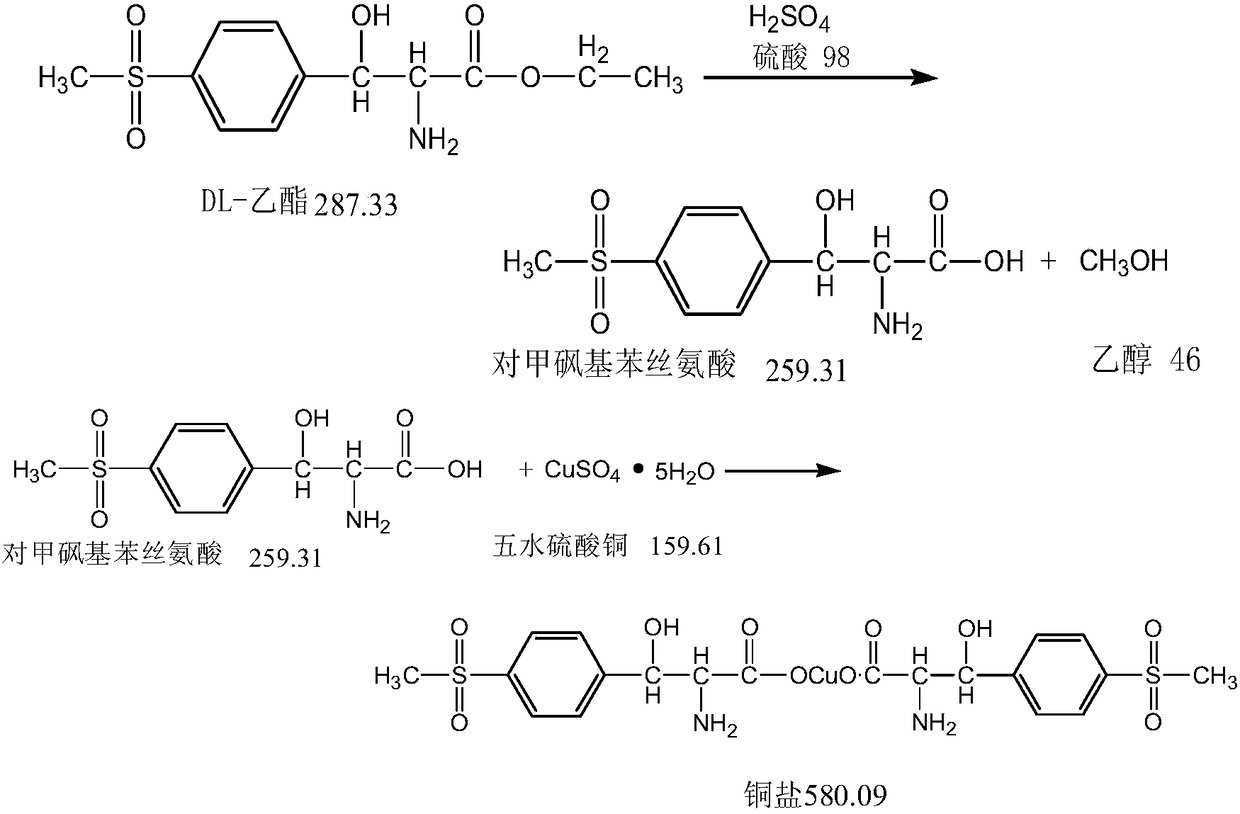
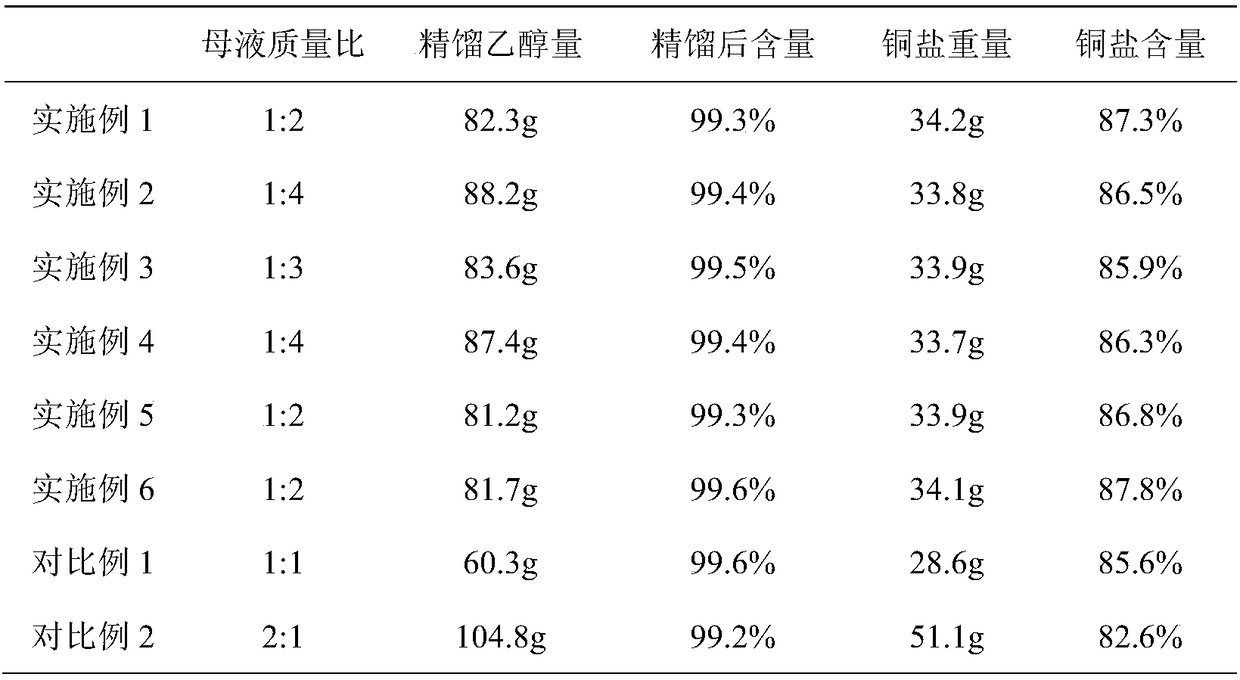
[0031] Add 300g of the esterification reaction mother liquor and 600g of the free D,L-threo-p-thymphenylphenylserine ethyl ester mother liquor into a 1000ml three-necked flask. After stirring, turn on the heating to 70°C for distillation, distill to 105°C, and ethanol Distillation column rectification; continue to add 5g of activated carbon to the mother liquor at 105°C, keep it for 2 hours for hydrolysis and decolorization, cool down to 30°C, filter, add 16g of copper sulfate pentahydrate to the filtrate, add liquid alkali dropwise to adjust PH=5, stir for 1 hour, Filter and dry. The results are shown in Table 1
Embodiment 2
[0033] Add 300g of the esterification reaction mother liquor and 1200g of the free D,L-threo-p-thymphenylphenylserine ethyl ester mother liquor to a 2000ml three-necked flask. After stirring, turn on the heating to 70°C for distillation, and then distill to 105°C. Rectification in the distillation column; continue to add 7.5g of activated carbon to the mother liquor at 110°C, keep it for 2 hours for hydrolysis and decolorization, cool down to 30°C, filter, add 16g of copper sulfate pentahydrate to the filtrate, add liquid alkali dropwise to adjust the pH to 5, and stir for 1 hour , filtered and dried. The results are shown in Table 1
Embodiment 3
[0035] Add 300g of esterification reaction mother liquor and 900g of free D,L-threo-p-thymphenylphenylserine ethyl ester mother liquor to a 2000ml three-neck flask, after stirring, turn on the heating to 70°C for distillation, then distill to 105°C, and ethanol Distillation column rectification; continue to add 6g of activated carbon to the mother liquor at 110°C, keep it for 2 hours for hydrolysis and decolorization, cool down to 30°C, filter, add 16g of copper sulfate pentahydrate to the filtrate, add liquid alkali dropwise to adjust PH=6.5, stir for 1 hour, Filter and dry. The results are shown in Table 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com