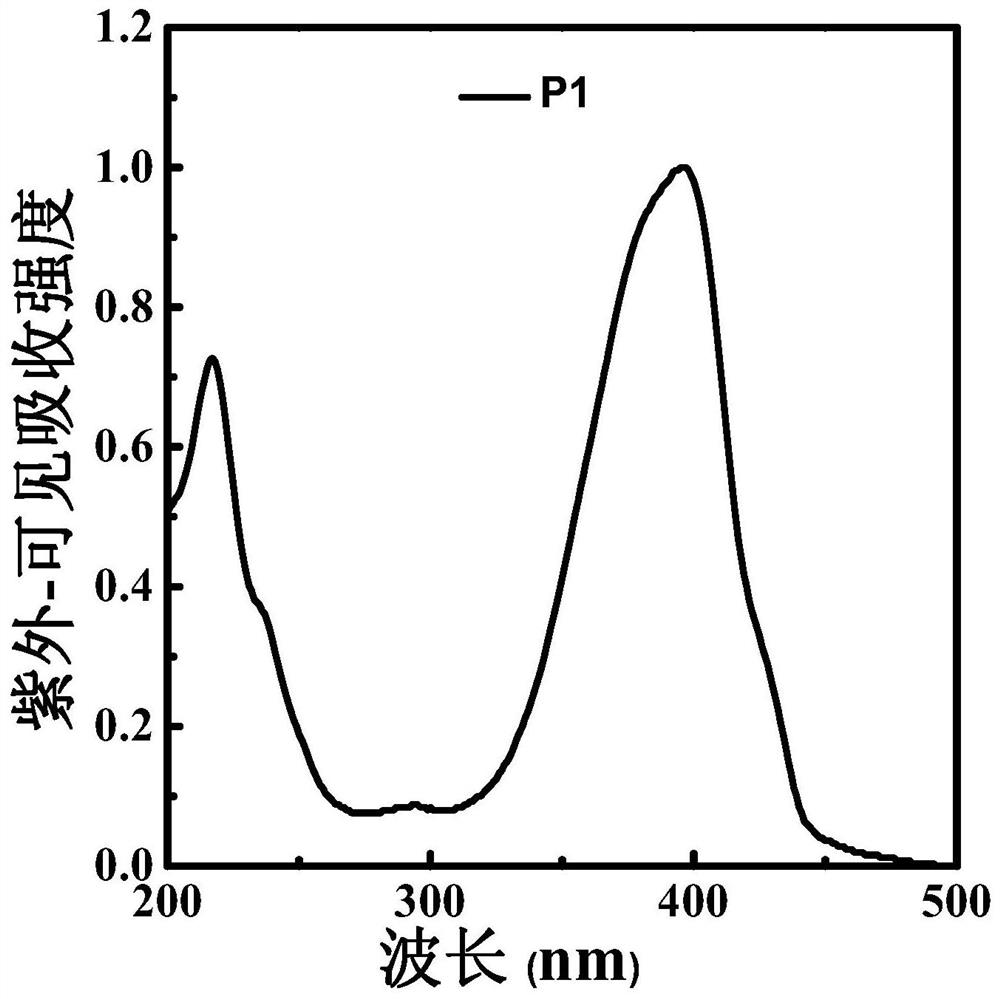
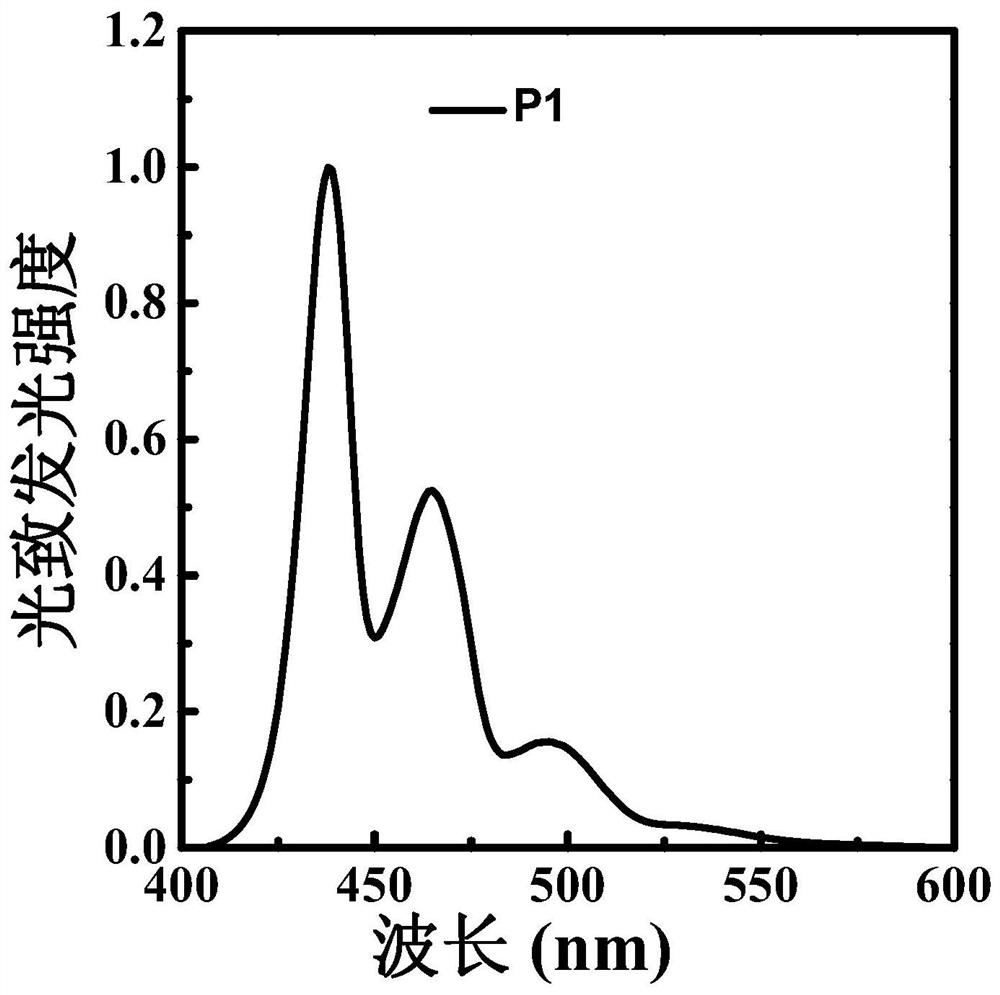
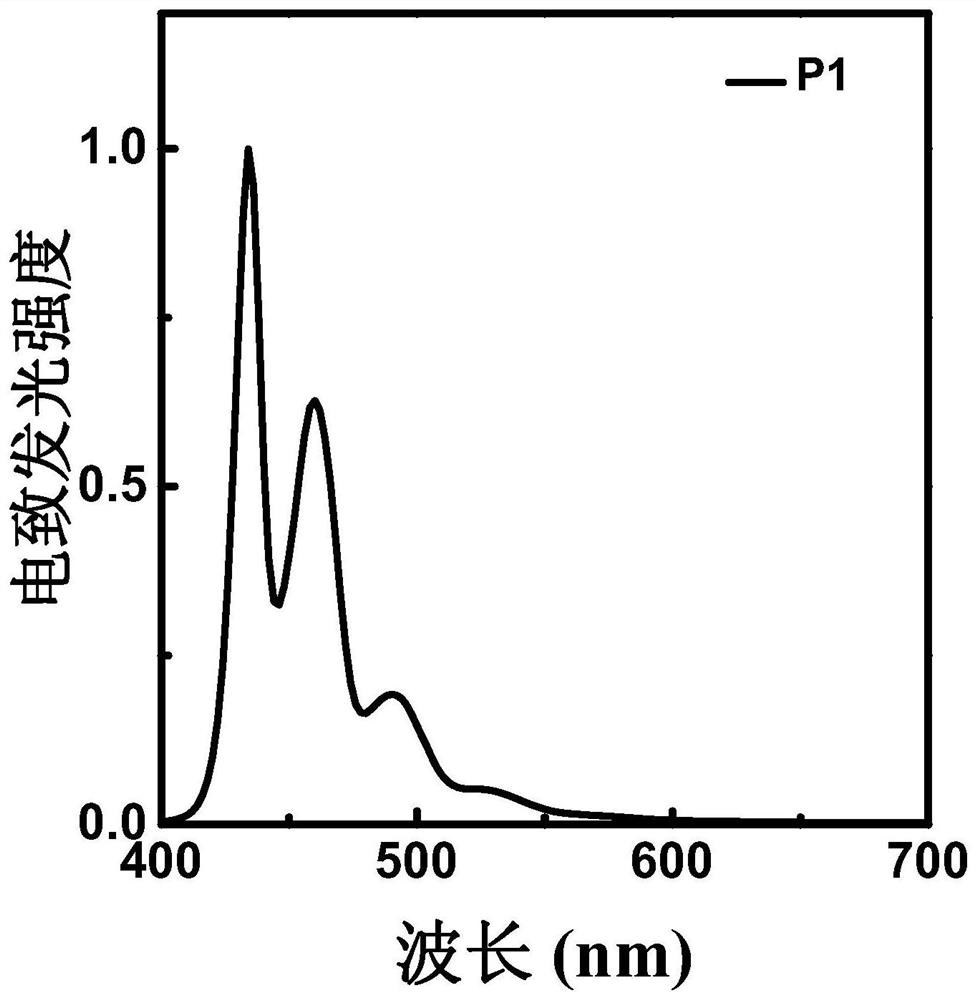
Polyfluorene derivative, light-emitting layer of light-emitting diode and preparation method thereof
A technology of light-emitting diodes and derivatives, applied in semiconductor/solid-state device manufacturing, electric solid-state devices, semiconductor devices, etc., can solve the problem of reducing hole transport performance, unbalanced carrier transport, limited device efficiency and stability, etc. problems, to achieve the effect of improving luminous efficiency, carrier transport balance, and maintaining color purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Preparation of compound M1
[0037] (1) preparation of compound 1: in a 100ml there-necked flask, add dibenzothiophene (11g, 60mmol), iron powder (0.17g, 3mmol) and elemental bromine (3.1mL, 60mmol), under nitrogen atmosphere, stirring reaction 16 at room temperature hours, then the reaction was quenched with aqueous sodium bisulfite, followed by three extractions with dichloromethane, and the solvent was removed under reduced pressure to give the crude product, which was then purified by column in about 80% yield.
[0038] (2) Preparation of compound 2: under nitrogen protection, add compound 1 (3.3g, 12.5mmol) to a 100ml there-necked flask, dissolve in dry tetrahydrofuran, then add butyllithium (3.3g, 12.5mmol), under nitrogen atmosphere , stirred at -78 degrees Celsius for 2 hours, then added 1-bromo-n-hexane (7.4g, 45mmol) to continue the reaction for 1 hour, then added water to quench the reaction, then extracted three times with dichloromethane, and removed the so...
Embodiment 1
[0053] Preparation of polymer P1
[0054] Synthesis of polymer P1: under nitrogen protection, 2,7-bis(4,4,5,5-tetramethyl-1,3-dioxo-2-boranyl)-9,9-bis(4 -(2-Ethylhexaneoxy)phenyl)fluorene (248.0 mg, 0.3 mmol), 2,7-dibromo-9,9-bis(4-(2-ethylhexaneoxy)phenyl) ) fluorene (175.8 mg, 0.24 mmol), and compound M3 (48.6 mg, 0.06 mmol) were dissolved in 10 mL of toluene, and then tetraethylhydroxylamine aqueous solution (1 ml, wt%=25%), palladium acetate (1 mg) and tris Cyclohexylphosphine (2mg); heated to 80°C for reaction for 24 hours, then added phenylboronic acid (20mg) to cap for 6 hours, and then added bromobenzene (0.2ml) at 80°C to cap for 6 hours; the reaction stopped, after cooling, the organic The phase was precipitated in methanol (200ml), filtered and dried. The crude product was extracted with methanol, acetone, and n-hexane successively. The polymer was dissolved in toluene, and toluene was used as the eluent to perform column chromatography with neutral alumina. Purif...
Embodiment 2
[0061] Preparation of polymer P2
[0062] The synthesis conditions of polymer P2 are the same as those of polymer P1, the difference is:
[0063] Polymer P2: 2,7-bis(4,4,5,5-tetramethyl-1,3-dioxo-2-boranyl)-9,9-bis(4-(2-ethylhexyl) Alkoxy)phenyl)fluorene (248.0mg, 0.3mmol), 2,7-dibromo-9,9-bis(4-(2-ethylhexaneoxy)phenyl)fluorene (175.8mg, 0.24 mmol), and compound M1 (41.7 mg, 0.06 mmol). GPC: Mn=97KDa, PDI=3.21.
[0064]
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com