Metformin hydrochloride enteric-coated sustained-release pellets and preparation method thereof
A technology of metformin hydrochloride enteric and metformin hydrochloride, which is applied in the field of metformin hydrochloride sustained-release pellets and its preparation, can solve problems such as adverse reactions and non-absorption by the human body, so as to avoid gastric irritation, improve medication compliance, and reduce side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
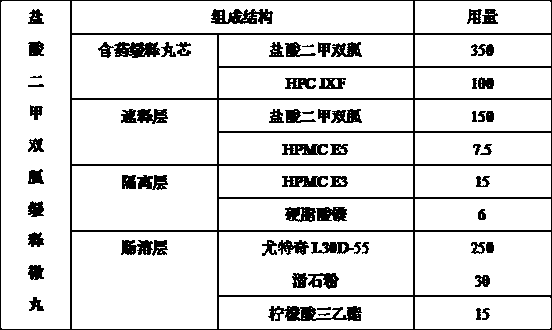
[0035] Prescription
[0036]
[0037]The preparation method is as follows:
[0038] 1) Raw and auxiliary materials are crushed and sieved
[0039] Metformin hydrochloride is pulverized on a pulverizer and sieved; the slow-release material is sieved on a vibrating sieve; raw materials and auxiliary materials are weighed according to the prescription.
[0040] 2) Wet mixing granulation
[0041] Put the weighed metformin hydrochloride and HPMC K100M into the wet granulator in sequence, mix at low speed for 600 seconds, atomize and add 20% of the above solid weight purified water, finish adding within 90 seconds, continue to stir for 30 seconds, and start at the same time Cutter at low speed to make wet granules and discharge.
[0042] 3) Extrusion, spheronization, drying, sieving
[0043] Put the wet granules into the rotary granulator respectively, select a screen with an aperture of 0.8mm, the extrusion speed is 70rpm, the spheronization speed is 550rpm, the spheronizati...
Embodiment 2
[0052] Prescription
[0053]
[0054] 1) Raw and auxiliary materials are crushed and sieved
[0055] Metformin hydrochloride is pulverized on a pulverizer and sieved; the slow-release material is sieved on a vibrating sieve; raw materials and auxiliary materials are weighed according to the prescription.
[0056] 2) Wet mixing granulation
[0057] Put the weighed metformin hydrochloride and HPC JXF into the wet granulator in sequence, mix at a low speed for 600 seconds, atomize and add 20% of the above solid weight purified water, finish adding within 120 seconds, continue to stir for 30 seconds, and start at the same time Cutter at low speed to make wet granules and discharge.
[0058] 3) Extrusion, spheronization, drying, sieving
[0059] Put the wet granules into the rotary granulator respectively, select a screen with an aperture of 0.8mm, the extrusion speed is 70rpm, the spheronization speed is 500rpm, the spheronization time is 3min, and the strips with uniform le...
Embodiment 3
[0068] In vitro dissolution test
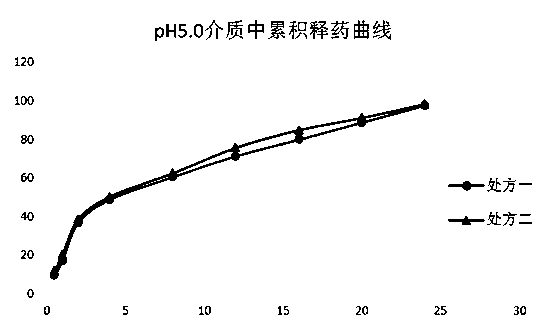
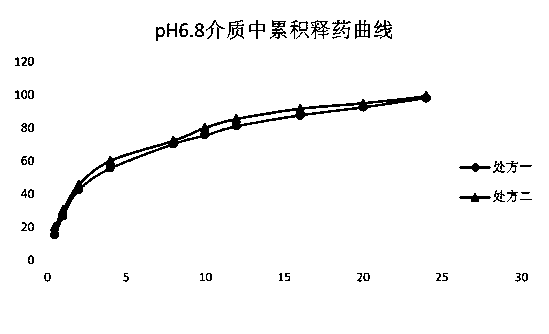
[0069] Prepare pH1.0, pH5.0 and pH6.8 solutions according to the regulations of Chinese Pharmacopoeia. The prepared pellets release less than 2% after 100rpm in pH1.0 medium for 2 hours, and the acid resistance is good, which meets the requirements.
[0070] The release situation in pH5.0 and pH6.8 media is as follows figure 1 and figure 2 As shown, it can be seen from the in vitro dissolution results that the acid resistance of the prepared metformin enteric-coated pellets meets the requirements, and can reach about 30% after rapid release in pH5.0 and pH6.8 media, and then continue to release slowly Maintained at 24h to release completely.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com