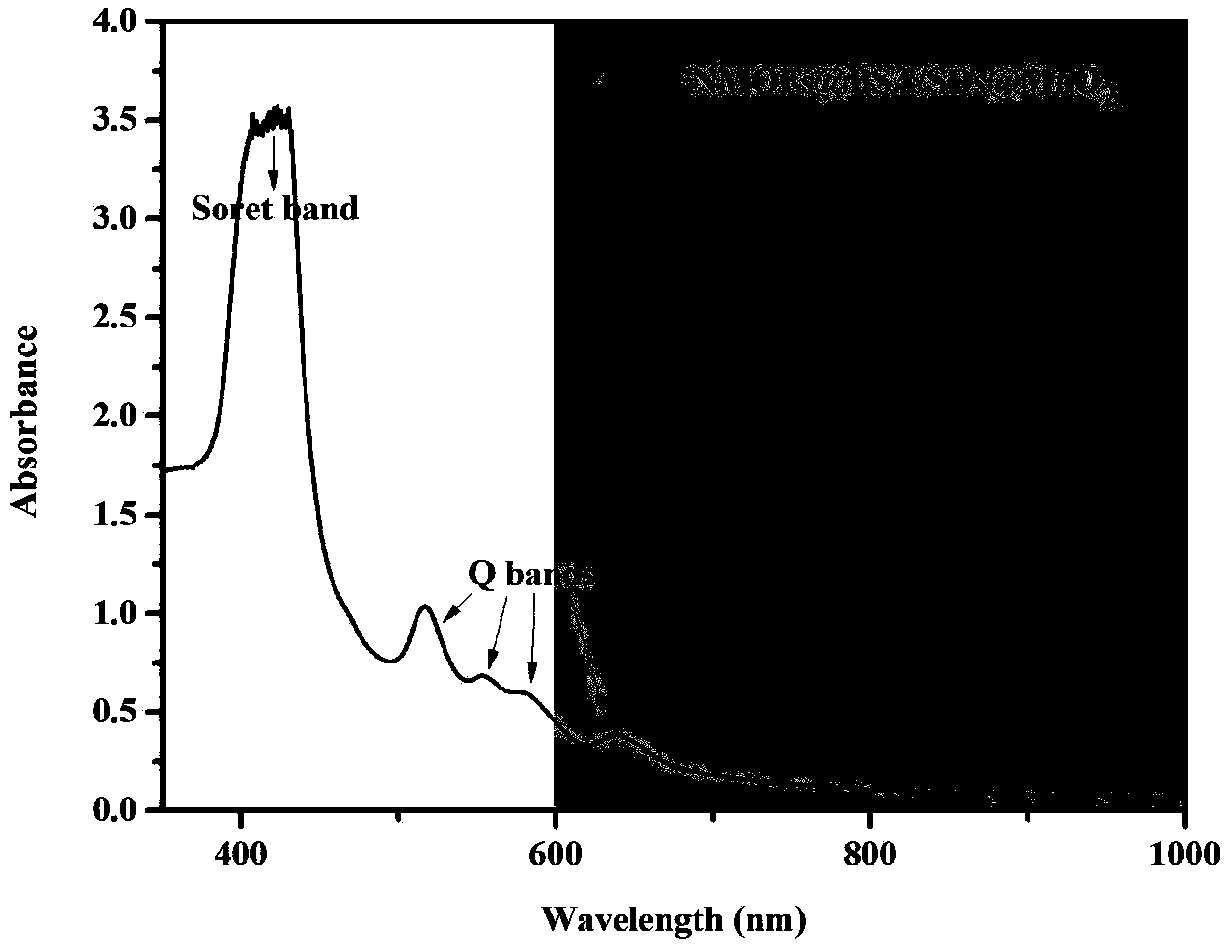
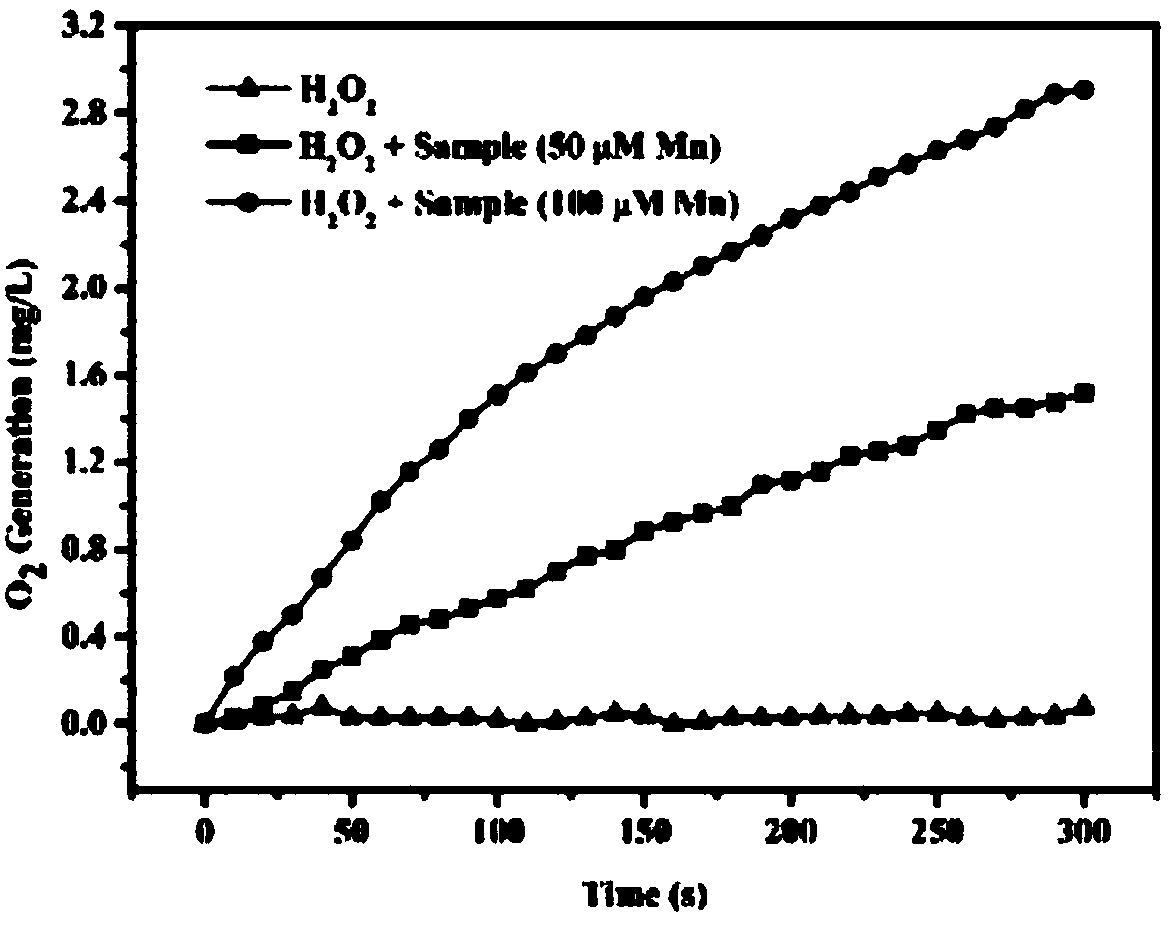
Nano carrier for tumor photo-dynamics therapy (PDT) and preparation method thereof
A photodynamic therapy, nanocarrier technology, applied in photodynamic therapy, nanotechnology for sensing, preparations for in vivo experiments, etc., can solve the problem of reduced oxygen concentration, low photodynamic therapy efficiency, and low ROS range and other problems to achieve the effect of increasing oxygen content, enhancing photodynamic therapy, good biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. In a 250mL flask, 2mLFe 3+ The solution (0.0125mM) was added to 10mL of DMF, then 2mL of TCPP solution (0.0125mM) was added dropwise, and 0.4mL of acetic acid was added to adjust the pH of the mixed solution to an acidic environment. After stirring vigorously at room temperature for 2 hours, the mixed solution was heated to 80°C and stirred slowly for 24 hours. After the reaction, the solution was naturally cooled to room temperature, centrifuged, washed with DMF, ethanol, and deionized water to remove residual solvents, and the purified NMOFs were stored at 4 °C. All reactions were performed in the dark.
[0026] 2. Take 2 mL of BSA solution (0.1 mM) and add it to 800 microliters of SDs solution (1 mM), and stir at room temperature for 12 hours to obtain a BSA / SDs mixture. 100 mg of NMOFs prepared in S1 were ultrasonically dispersed in deionized water for 2 hours, then 40 mg of EDC and 30 mg of NHS were added immediately under magnetic stirring at 40 °C, and the B...
Embodiment 2
[0029] Step 1. In a 250mL flask, 2mLFe 3+The solution (0.0127mM) was added to 15mL of DMF, then 2mL of TCPP solution (0.0127mM) was added dropwise, and 0.5mL of acetic acid was added to adjust the pH of the mixed solution to an acidic environment. After stirring vigorously at room temperature for 2 hours, the mixed solution was heated to 80°C and stirred slowly for 24 hours. After the reaction, the solution was naturally cooled to room temperature, centrifuged, washed with DMF, ethanol, and deionized water to remove residual solvents, and the purified NMOFs were stored at 4 °C. All reactions were performed in the dark.
[0030] Step 2. Take 2 mL of BSA solution (0.1 mM) and add it to 800 microliters of SDs solution (1 mM), and stir at room temperature for 12 hours to obtain a BSA / SDs mixture. 100 mg of NMOFs prepared in S1 were ultrasonically dispersed in deionized water for 2 hours, then 40 mg of EDC and 30 mg of NHS were added immediately under magnetic stirring at 40 °C, ...
Embodiment 3
[0033] 1. In a 250mL flask, 2mLFe 3+ The solution (0.013mM) was added to 15mL of DMF, then 2mL of TCPP solution (0.013mM) was added dropwise, and 0.5mL of acetic acid was added to adjust the pH of the mixed solution to an acidic environment. After stirring vigorously at room temperature for 2 hours, the mixed solution was heated to 80°C and stirred slowly for 24 hours. After the reaction, the solution was naturally cooled to room temperature, centrifuged, washed with DMF, ethanol, and deionized water to remove residual solvents, and the purified NMOFs were stored at 4 °C. All reactions were performed in the dark.
[0034] 2. Take 2 mL of BSA solution (0.1 mM) and add it to 800 microliters of SDs solution (1 mM), and stir at room temperature for 12 hours to obtain a BSA / SDs mixture. 100 mg of NMOFs prepared in S1 were ultrasonically dispersed in deionized water for 2 hours, then 40 mg of EDC and 30 mg of NHS were added immediately under magnetic stirring at 40 °C, and the BSA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com