Esterified weak acid catalyst for synthesizing 2,2,4-trimethylpentanediol diisobutyrate
A technology of pentanediol diisobutyrate and catalyst, which is applied in physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, carboxylate preparation, etc. Uniformity, high catalyst preparation cost, etc., to achieve the effect of high esterification activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
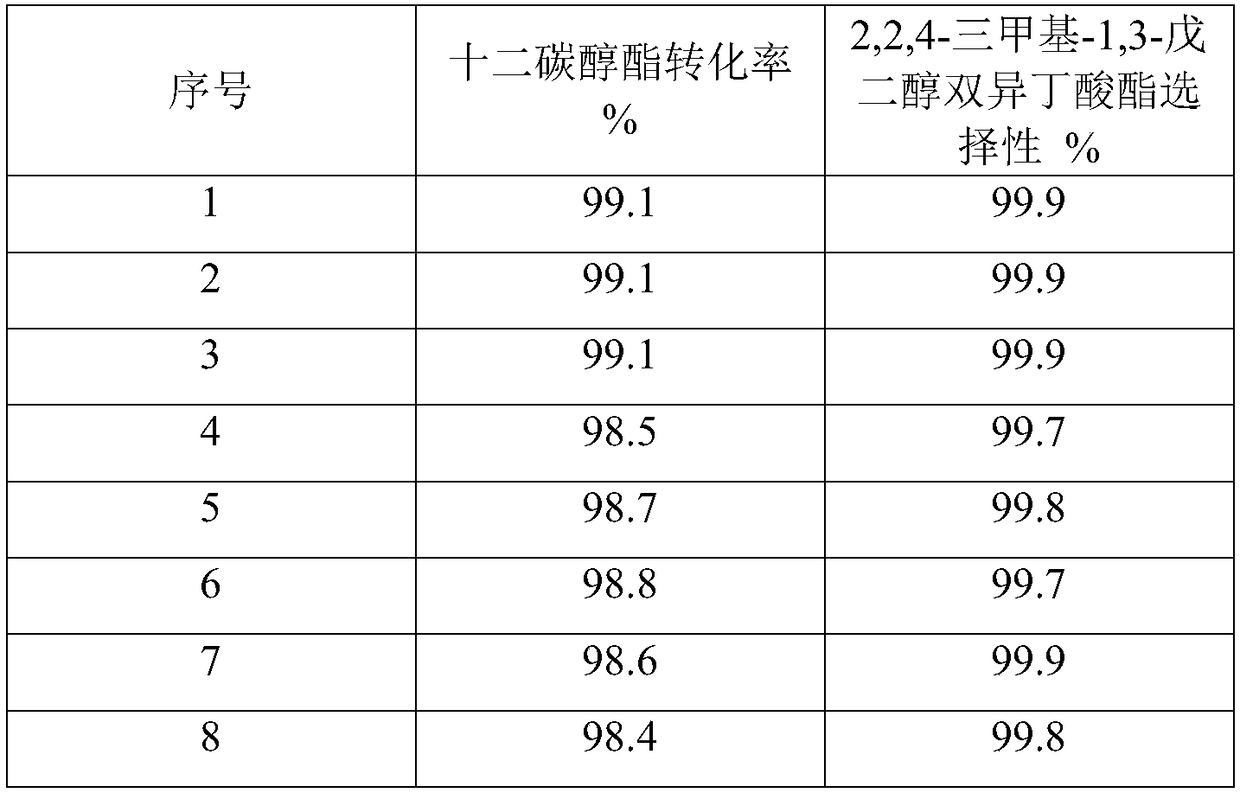
Examples
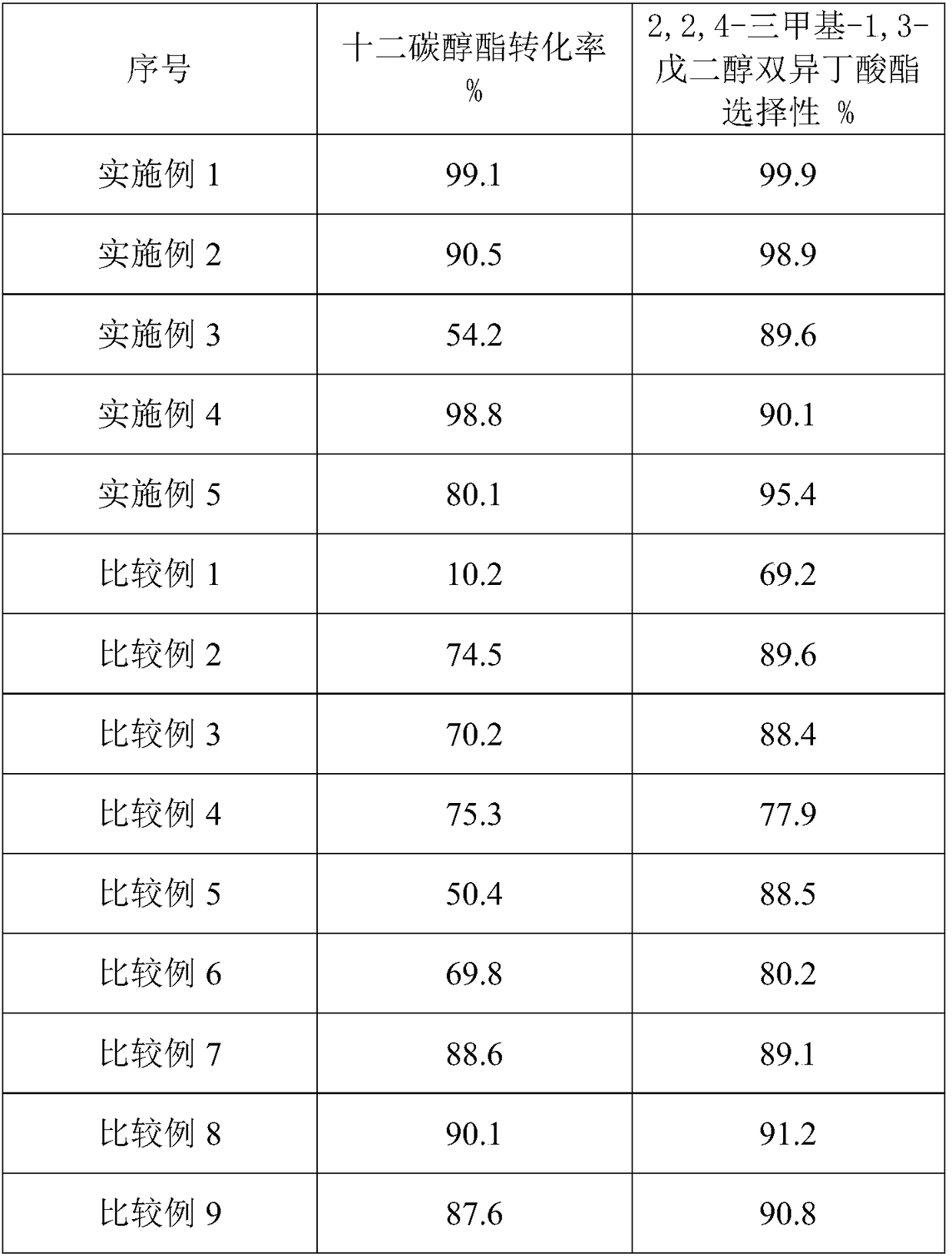
Embodiment 1
[0020] Put 10g of raw soil into a 250mL round bottom flask, add 200mL of 2mol / L hydrochloric acid solution, stir in a water bath at 70°C for 24h, filter and wash until there is no Cl - . Add 20g of hydrochloric acid-treated attapulgite into 100mL of deionized water, stir for 2h, dissolve 6.2g of ammonium vanadate in deionized water and add dropwise to the hydrochloric acid-treated attapulgite solution, stir for 3h, vacuum at 85°C Dry in an oven, and bake in a muffle furnace at 500°C for 3h. , after testing, vanadium accounts for 10% of the mass of the modified attapulgite.
[0021] Weigh a certain amount of isobutyric acid and dodecyl alcohol ester, the mass ratio of isobutyric acid and dodecyl alcohol ester is 1:1, add 1% to 2% (based on the total weight of raw materials) vanadium composite modified uneven Pyrolith solid catalyst, stirring, stably controlling the reaction temperature to rise to 155°C, condensing and refluxing to separate water, the reaction time continued f...
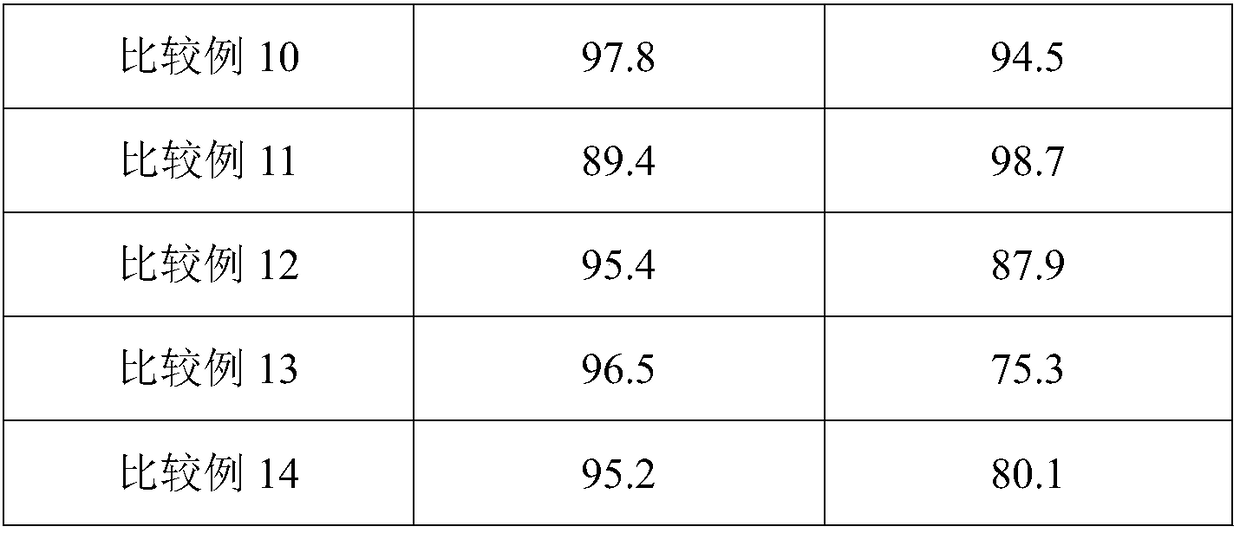
Embodiment 2
[0023] Treat attapulgite with 20 g of nitric acid instead of 20 g of hydrochloric acid in Example 1, and the other steps are the same as in Example 1.
Embodiment 3
[0025] The attapulgite was treated with 20 g of attapulgite instead of 20 g of hydrochloric acid in Example 1, and the other steps were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com