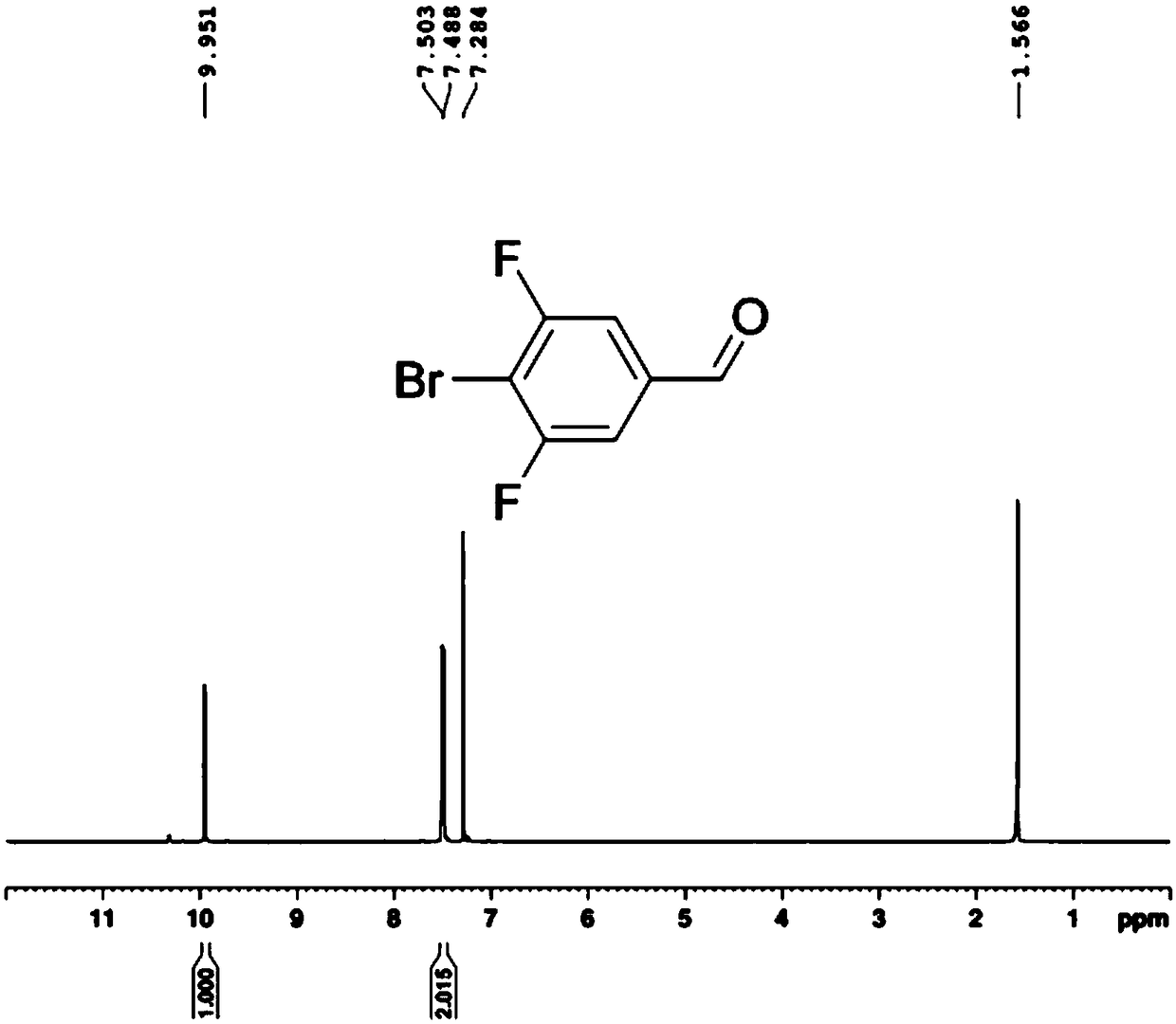
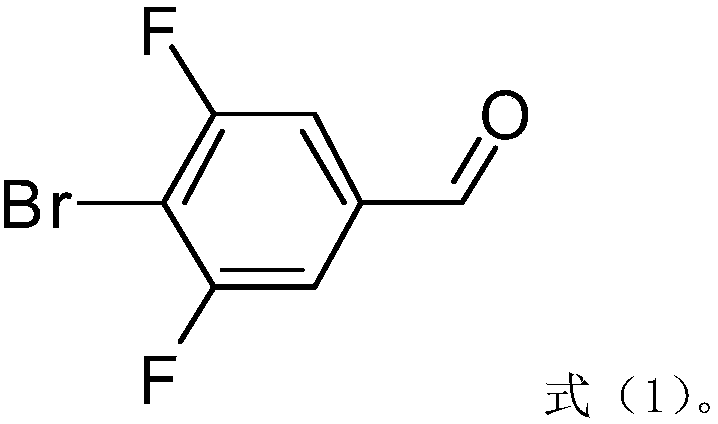
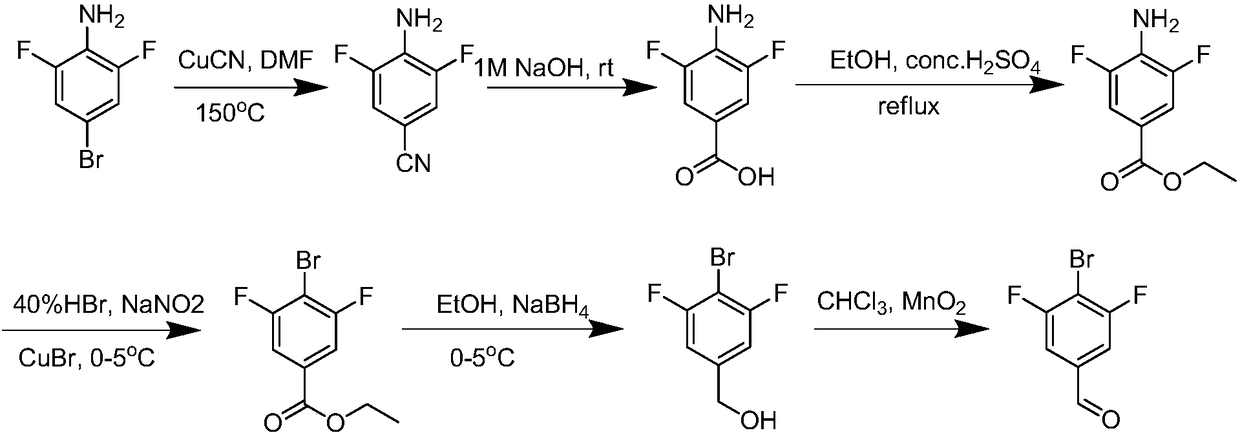
Bromofluoro polysubstituted benzaldehyde derivative and preparation method
A technology of bromobenzaldehyde and bromoaniline, applied in the field of pharmaceutical intermediates 3, can solve the problems of short reaction time, cannot be promoted, cannot have higher yield reaction time, etc., and achieves the effects of easy operation, high economic value, and saving raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step S1: Prepare 90g of 2,6-difluoro-4-bromoaniline, 900ml of N,N-dimethylformamide and 124g of cuprous cyanide as raw materials, and select a 2L four-necked flask as the reaction vessel , Blow in nitrogen protection, add the aforementioned 2,6-difluoro-4-bromoaniline to all the N,N-dimethylformamide in the four-neck flask, then add all the cuprous cyanide, and reflux for 18h After confirming the completion of the reaction by TLC detection, cool the reaction mixture to room temperature, prepare a large open container to hold 2.5L of 15% ammonia water, pour the cooled reaction mixture, extract 3 times with appropriate ethyl acetate, and combine The organic phase was washed three times with 400ml of saturated brine, dried with 90g of anhydrous sodium sulfate, and concentrated to dryness under vacuum to obtain the crude product of step S1; the crude product of step S1 was dissolved in 0.9L of dichloromethane, and 100- 140g 200 mesh silica gel, after mixing uniformly, remove...
Embodiment 2
[0029] Step S1: Prepare 100g of 2,6-difluoro-4-bromoaniline, 1000ml of N,N-dimethylformamide and 128g of cuprous cyanide as raw materials, and select a 2.5L four-necked flask as the reaction The container is protected by nitrogen, and the aforementioned 2,6-difluoro-4-bromoaniline is added to all the N,N-dimethylformamide in the four-neck flask, and then all of the cuprous cyanide is added, and refluxed 20h, after confirming the completion of the reaction by TLC, cool the reaction mixture to room temperature, prepare a large open container to hold 3L of 15% ammonia water, pour the cooled reaction mixture, extract 4 times with appropriate amount of ethyl acetate, and combine The organic phase was washed three times with 500ml of saturated brine, dried with 100g of anhydrous sodium sulfate, and concentrated to dryness under vacuum to obtain the crude product of step S1; dissolve the crude product of step S1 with 1L of dichloromethane, and add 100-200 150g mesh silica gel, after m...
Embodiment 3
[0036] Step S1: Prepare 110g of 2,6-difluoro-4-bromoaniline, 1100ml of N,N-dimethylformamide and 132g of cuprous cyanide as raw materials, and select a 3L four-necked flask as the reaction vessel , Blow in nitrogen protection, add the aforementioned 2,6-difluoro-4-bromoaniline to all the N,N-dimethylformamide in the four-neck flask, then add all the cuprous cyanide, and reflux for 22h After confirming the completion of the reaction by TLC detection, cool the reaction mixture to room temperature, prepare a large open container to hold 3.5L of 15% ammonia water, pour the cooled reaction mixture, extract 5 times with appropriate ethyl acetate, and combine The organic phase was washed three times with 600ml of saturated brine, dried with 110g of anhydrous sodium sulfate, and concentrated to dryness under vacuum to obtain the crude product of step S1; the crude product of step S1 was dissolved in 1.1L of dichloromethane and added 100- 160g 200 mesh silica gel, after mixing uniformly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com