Production process for synthesizing tricyclazole by using solid super acid
A solid super acid and production process technology, applied in the field of pesticides, can solve the problems of large environmental pollution, low utilization rate of formic acid, and low recovery rate, and achieve the effects of reducing pollution, eliminating recovery and purification processes, and improving utilization rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
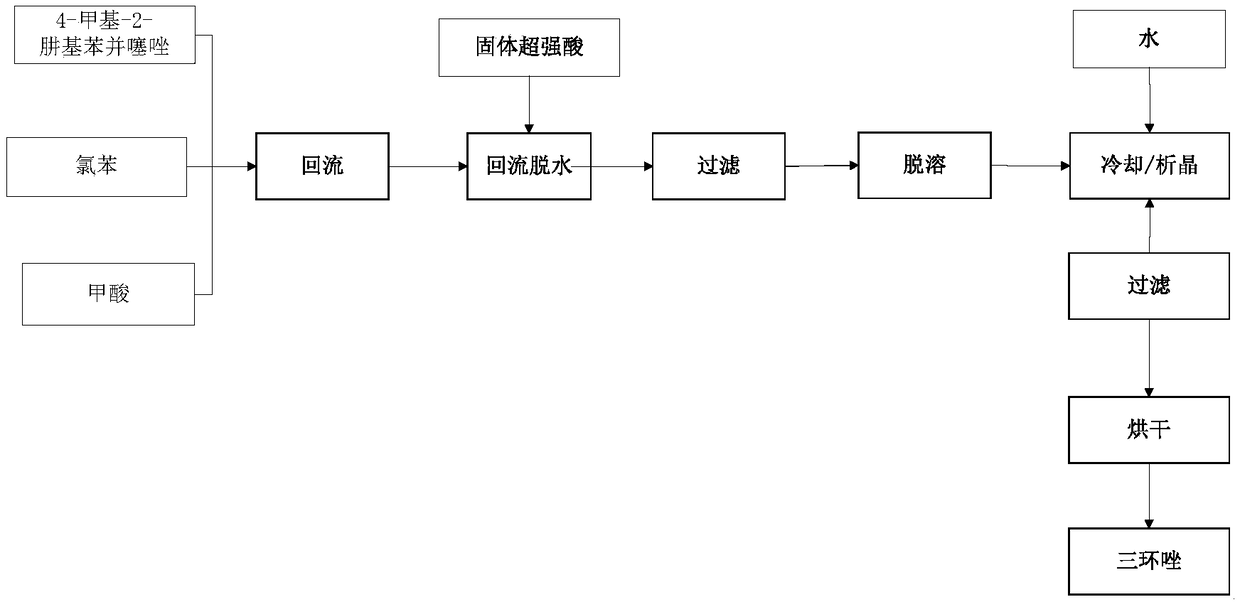
[0028] A production process for synthesizing tricyclazole using solid superacid, using intermediate 4-methyl-2-hydrazinobenzothiazole as raw material, formic acid as cyclizing agent, chlorobenzene as solvent, and solid superacid as catalyst to synthesize tricyclazole Azole; the solid superacid is perfluorosulfonic acid resin; 179kg of 4-methyl-2-hydrazinobenzothiazole, 537kg of chlorobenzene, 172.5kg of 40% formic acid, and 8.95kg of solid superacid.
[0029] The production technique of using solid superacid to synthesize tricyclazole comprises the following steps:
[0030] Step S1, mixing the 4-methyl-2-hydrazinobenzothiazole with the chlorobenzene, then adding the formic acid, and raising the temperature to reflux until the 4-methyl-2-hydrazinobenzothiazole is completely converted Forming an oxime-based intermediate; wherein, the heating temperature for heating and refluxing is 136°C; the time for heating and refluxing is 0.5h;
[0031] Step S2, adding the solid superacid t...
Embodiment 2
[0033] A production process for synthesizing tricyclazole using solid superacid, using intermediate 4-methyl-2-hydrazinobenzothiazole as raw material, formic acid as cyclizing agent, chlorobenzene as solvent, and solid superacid as catalyst to synthesize tricyclazole Azole; the solid superacid is perfluorosulfonic acid resin; 179kg of 4-methyl-2-hydrazinobenzothiazole, 626.5kg of chlorobenzene, 53.67kg of 90% formic acid, and 17.9kg of solid superacid.
[0034] The production technique of using solid superacid to synthesize tricyclazole comprises the following steps:
[0035] Step S1, mixing the 4-methyl-2-hydrazinobenzothiazole with the chlorobenzene, then adding the formic acid, and raising the temperature to reflux until the 4-methyl-2-hydrazinobenzothiazole is completely converted Forming an oxime-based intermediate; wherein, the heating temperature for heating and refluxing is 139°C; the time for heating and refluxing is 2h;
[0036] In step S2, add the solid superacid t...
Embodiment 3
[0038] A production process for synthesizing tricyclazole using solid superacid, using intermediate 4-methyl-2-hydrazinobenzothiazole as raw material, formic acid as cyclizing agent, chlorobenzene as solvent, and solid superacid as catalyst to synthesize tricyclazole Azole; the solid superacid is perfluorosulfonic acid resin; 179kg of 4-methyl-2-hydrazinobenzothiazole, 600kg of chlorobenzene, 85.43kg of 70% formic acid, and 12kg of solid superacid.
[0039] The production technique of using solid superacid to synthesize tricyclazole comprises the following steps:
[0040] Step S1, mixing the 4-methyl-2-hydrazinobenzothiazole with the chlorobenzene, then adding the formic acid, and raising the temperature to reflux until the 4-methyl-2-hydrazinobenzothiazole is completely converted Forming an oxime-based intermediate; wherein, the heating temperature for heating and refluxing is 138°C; the time for heating and refluxing is 1.5h;
[0041]In step S2, add the solid superacid to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com