Peptide-drug co-assembly method for constructing reduction-responsive anticancer nanomedicine
A nanomedicine and co-assembly technology, applied in the field of nanomaterial technology and biomedical materials, can solve the problems of high toxicity and high synthesis cost, and achieve the effects of low biological toxicity, easy degradation and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
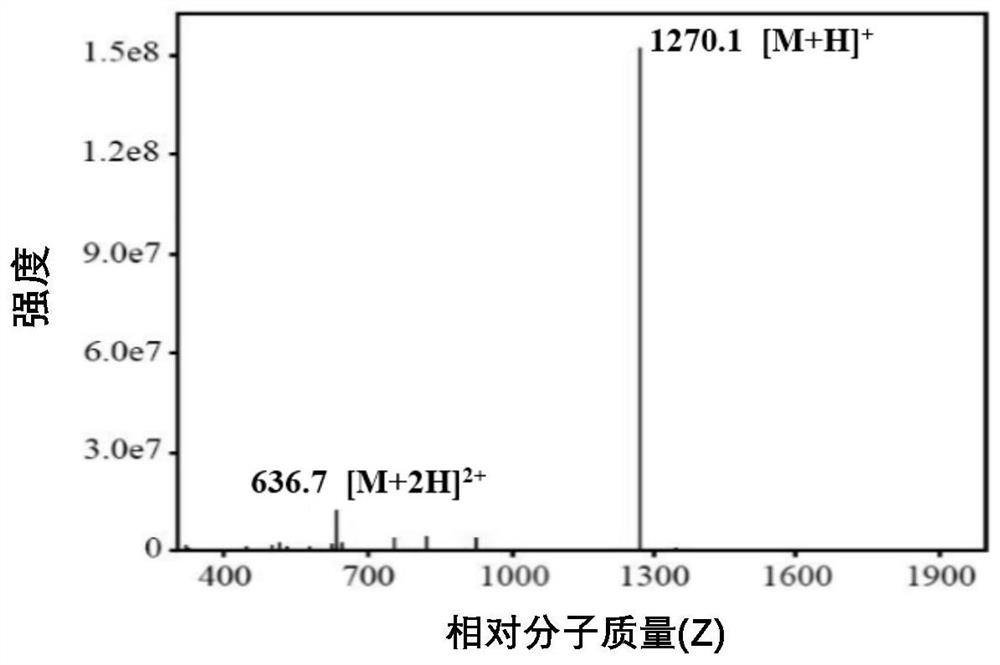
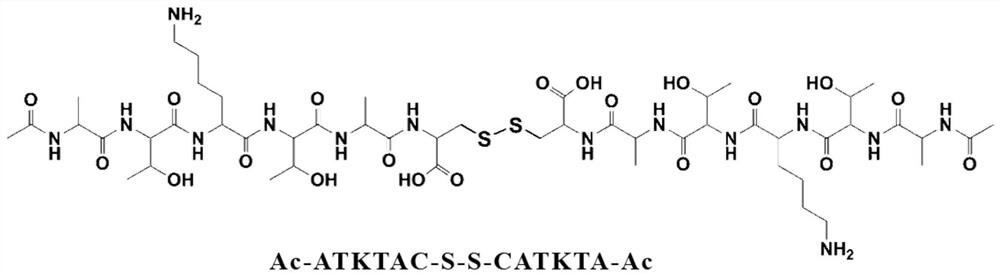
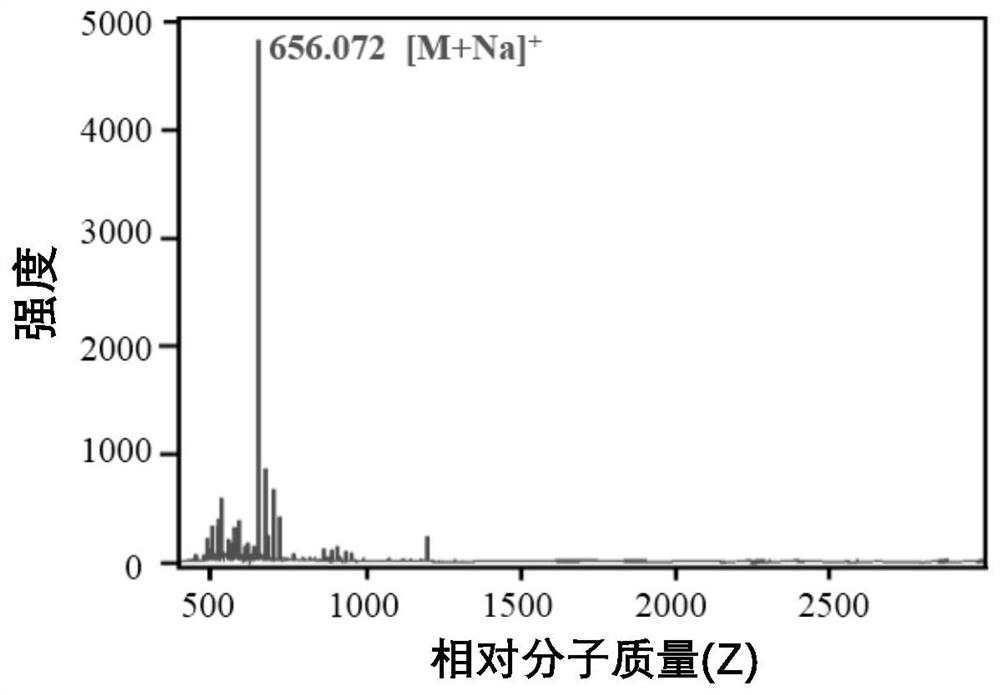
Image
Examples
Embodiment 1
[0034] 1) Weigh the polypeptide and curcumin (CCM) and dissolve them in tetramethyl sulfoxide (DMSO), the concentration of the polypeptide is 1mg / 20μL DMSO, the concentration of CCM is 1mg / 30μL DMSO, according to the molar ratio of polypeptide:CCM=5:3 Mix the above two solutions, then add pH=5.5 phosphate buffer to dilute, the buffer concentration is 50mM, the final concentration of the diluted polypeptide is 0.5mg / mL, and the final concentration of curcumin is 0.3mg / mL;
[0035] 2) Incubating the mixed solution in step 1) at 16°C for 1 hour;
[0036] 3) After the incubation of the mixed solution in step 2) is completed, the mixed solution is centrifuged at 6000 rpm for 3 minutes to remove the precipitate, and the supernatant is taken to obtain the nanomedicine.
Embodiment 2
[0038] 1) Weigh the polypeptide and curcumin (CCM) and dissolve them in ethanol respectively, the concentration of the polypeptide is 1 mg / 5 μL ethanol, the concentration of CCM is 1 mg / 100 μL ethanol, mix the above two solutions according to the molar ratio of polypeptide:CCM=5:1, Then add citrate buffer with pH=6.5 for dilution, the buffer concentration is 30mM, the final concentration of the diluted polypeptide is 0.3mg / mL, and the final concentration of curcumin is 0.06mg / mL;
[0039] 2) Incubating the mixed solution in step 1) at 30° C. for 1 hour;
[0040]3) After the incubation of the mixed solution in step 2) is completed, the mixed solution is centrifuged at 4000 rpm for 10 minutes to remove the precipitate, and the supernatant is taken to obtain the nanomedicine.
Embodiment 3
[0042] 1) Weigh the polypeptide and 10-hydroxycamptothecin (HCPT) and dissolve them in methanol respectively. The concentration of the polypeptide is 1mg / 10μL methanol, and the concentration of CCM is 1mg / 60μL methanol. The two solutions were then diluted with a citrate buffer solution of pH=5, the buffer solution concentration was 45mM, the final concentration of the diluted polypeptide was 0.8mg / mL, and the final concentration of curcumin was 0.8mg / mL;
[0043] 2) Incubating the mixed solution in step 1) at 25°C for 2.5 hours;
[0044] 3) After the incubation of the mixed solution in step 2) is completed, the mixed solution is centrifuged at 5000 rpm for 8 minutes to remove the precipitate, and the supernatant is taken to obtain the nanomedicine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com