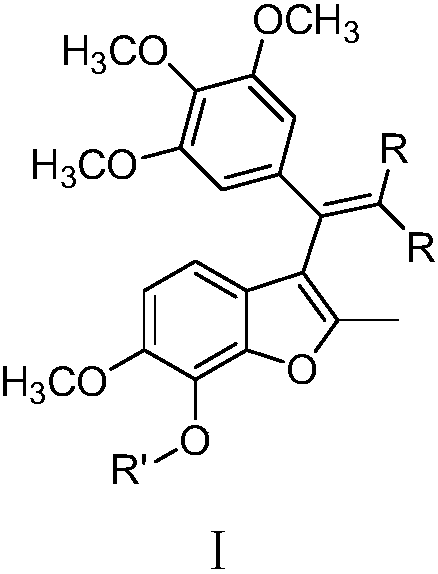
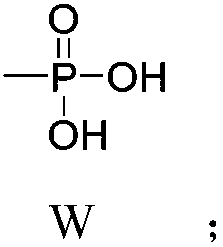
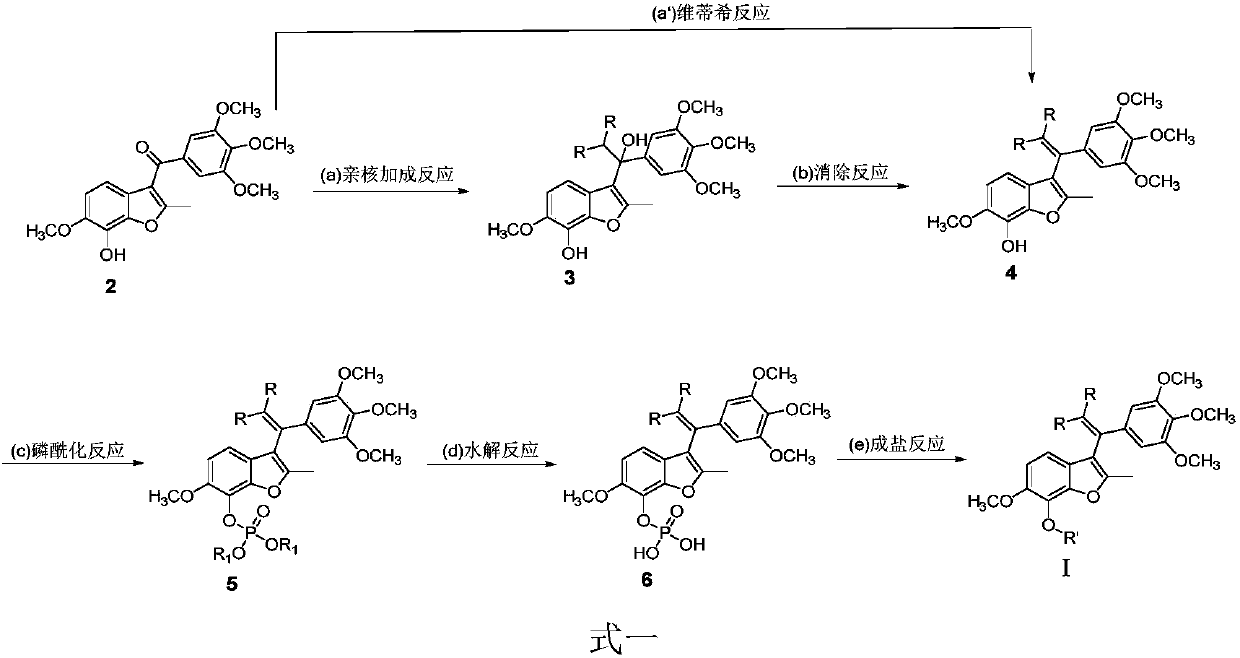
Substituted benzofuran derivative, preparation and application thereof in preparing antitumor drug
A technology of benzofuran and derivatives, applied in the field of medicinal chemistry, can solve the problems of limited clinical application and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1, 6-methoxy-2-methyl-3-(1-(3,4,5-trimethoxyphenyl)allyl-2,2-dideuterium)-benzofuran-7 -phenol, the synthesis of compound 4 shown in formula one
[0061]
[0062] Take deuteromethyltriphenylphosphine bromide (3.57g, 10mmol) in a three-necked flask, add anhydrous tetrahydrofuran 30mL under the protection of argon, drop to about -70°C, drop n-butyllithium solution (5mL, 11mmol , 2.2M hexane solution), after 1 hour, drop into 30 mL of anhydrous THF solution of BNC105 (1.87 g, 5 mmol), naturally return to room temperature at 25 ° C, stir until TLC shows that the raw materials are completely consumed, then drop to about 0 ° C, drop Quenched by adding 60 mL of ammonium chloride aqueous solution, the reaction solution was extracted twice with 180 mL of ethyl acetate (90 mL*2 times), combined with the organic phase of ethyl acetate, washed once with 70 mL of water, dried over anhydrous sodium sulfate, filtered, 45 °C Concentrate under reduced pressure on a rotary ev...
Embodiment 2
[0063] Example 2, 3-(2,2-dichloro-1-(3,4,5-trimethoxyphenyl)ethyl)-6-methoxy-2-methylbenzofuran-7-ol , the synthesis of compound 4 shown in formula one
[0064]
[0065]Take diethyl trichloromethylphosphonate (2.56g, 10mmol) in a three-necked flask, add 30mL of anhydrous tetrahydrofuran under the protection of argon, cool down to about -80°C, drop n-butyllithium solution (5mL, 11mmol, 2.2M hexane solution), after 1 hour, drop into 30 mL of anhydrous tetrahydrofuran solution of BNC105 (3.72 g, 10 mmol), naturally return to room temperature at 25 ° C, heat to reflux until TLC shows that the raw materials are completely consumed, and then drop to about 0 ° C, Drop in 60 mL of ammonium chloride aqueous solution to quench, the reaction solution was extracted twice with 160 mL of ethyl acetate (80 mL*2 times), the organic phase of ethyl acetate was combined, washed once with 60 mL of water, dried over anhydrous sodium sulfate, filtered, 45 ℃, vacuum degree 0.1MPa rotary evaporat...
Embodiment 3
[0066] Example 3, 3-(2,2-dichloro-1-(3,4,5-trimethoxyphenyl)ethyl)-6-methoxy-2-methylbenzofuran-7-ol , the synthesis of compound 4 shown in formula one
[0067]
[0068] Take benzyltriphenylphosphine bromide (4.33g, 10mmol) in a three-necked flask, add 30mL of anhydrous tetrahydrofuran under the protection of argon, lower it to about -70°C, drop n-butyllithium solution (5mL, 11mmol, 2.2 M hexane solution), after 1 hour, drop into 30 mL of anhydrous tetrahydrofuran solution of BNC105 (1.87 g, 5 mmol), naturally return to room temperature at 25 ° C, stir until TLC shows that the raw materials are completely consumed, then drop to about 0 ° C, and drop into 60 mL Ammonium chloride aqueous solution was quenched, the reaction solution was extracted twice with 120 ml of ethyl acetate (60 ml*2 times), the organic phase of ethyl acetate was combined, washed once with 50 ml of water, dried over anhydrous sodium sulfate, filtered, and at 45°C, Concentrate under reduced pressure with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com