Non-ionic emulsifier, epoxy emulsion, preparation method and application thereof
A non-ionic, epoxy emulsion technology, applied in the direction of epoxy resin coatings, coatings, anti-corrosion coatings, etc., can solve the problems of cumbersome preparation process, inability to apply metal welding anti-corrosion, complex components of epoxy emulsifier, etc. High corrosion potential, effect of inhibiting metal corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] This embodiment prepares non-ionic epoxy emulsion through the following steps
[0106] (1) In a 1000ml four-necked flask equipped with a thermometer, a nitrogen conduit, a stirrer, a water separator and an electric heating mantle, 580 g of polyethylene glycol monomethyl ether MPEG5000 (number-average molecular weight Mn=5000) was first dissolved in 100 ~110℃ through N 2 Purify for 30-60 minutes, then add 20g of methyltetrahydrophthalic anhydride (MTHPA) and 0.1% p-toluenesulfonic acid catalyst, maintain nitrogen flow, keep the reaction at 110°C for 4-5 hours, and measure the acid value ≤ 13.5mgKOH / g;
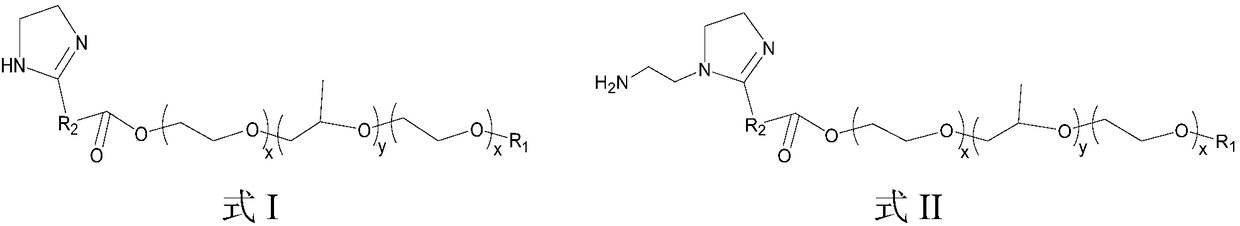
[0107] (2) Add 10g of ethylenediamine, 60g of toluene (it can be divided into two batches, and the water separator is filled with toluene in advance), react at 110-120°C for 2-3h, slowly raise the temperature to 210°C for 1-2h, and maintain 210°C Continue to react at ~220°C for 2~3h (water output is about 4g, primary amine value ≤5mgKOH / g), cool down to 160°C, release th...
Embodiment 2
[0114] (1) This step adopts the same reaction conditions as in Example 1, using 587g MPEG8000 (number-average molecular weight 8000) and 12.8g methyltetrahydrophthalic anhydride to prepare the half-ester, with an end point acid value≤8mgKOH / g;
[0115] (2) Add 10g of diethylenetriamine, 60g of toluene (it can be divided into two batches, and the water separator is filled with toluene in advance), react at 140-180°C for 2-3h, heat up to 210°C and maintain 210-220°C to continue the reaction 3h (water output is about 2.6g, primary amine value ≤11mgKOH / g), cool down to 160°C, release the solvent and by-products in the water separator, and vacuum extract for 1h;
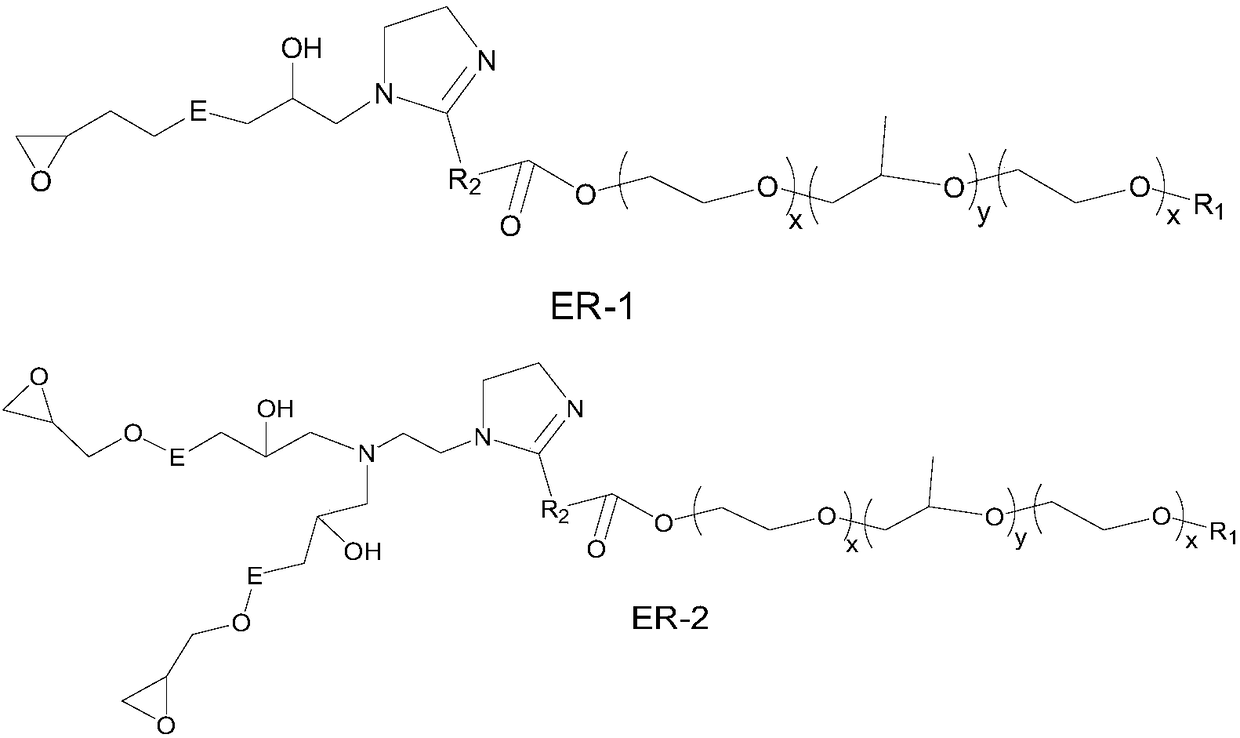
[0116] (3) Further lower the temperature to 85°C, add 162g of NPES-301 (EEW: 450-500) epoxy resin and 90g of propylene glycol methyl ether, and maintain the reaction at 85-90°C for 3h (tertiary amine value 12-16mgKOH / g), and obtain about 860g, an emulsifier with a solid content of 90%, named Ex-2 / ER-2;
[0117] (4) prepa...
Embodiment 3
[0121] (1) This step adopts the same reaction conditions as in Example 1, polyethylene glycol PEG6000 (number average molecular weight Mn=6000) 584g and 15.7g hexahydrophthalic anhydride are first half-esterified, and the terminal acid value is less than or equal to 10mgKOH / g;
[0122] (2) This step adopts the same reaction conditions as step (2) in Example 2, and diethylenetriamine is replaced by 8.5g ethylenediamine to prepare imidazoline, the water output is about 3.4g, and the primary amine value at the end is ≤5mgKOH / g;
[0123] (3) Further lower the temperature to 85°C, add 134g of NPES-302 (EEW: 600-700) epoxy resin and 90g of propylene glycol methyl ether, and maintain the reaction at 85-90°C for 3h (tertiary amine value 11-14mgKOH / g), and obtain about 837g, an emulsifier with a solid content of 90%, named Ex-3 / ER-1;
[0124] (4) prepare epoxy emulsion by following batching:
[0125]
[0126] Utilize the emulsification method among the embodiment 1, obtain solid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com