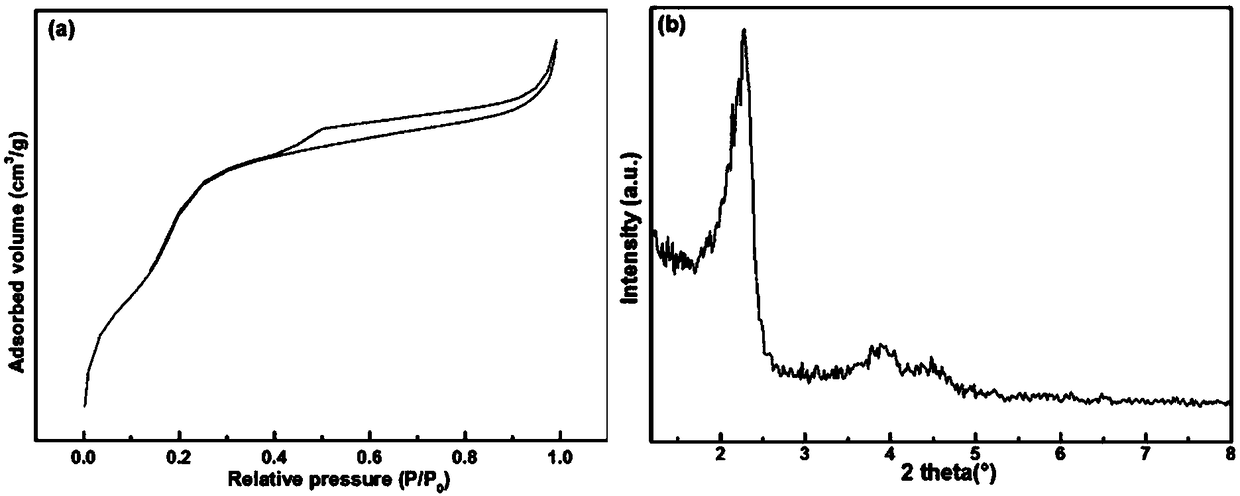
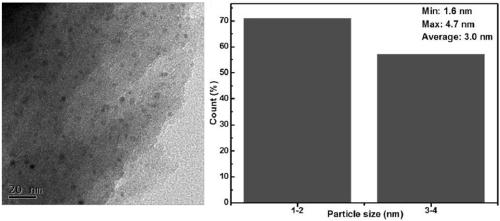
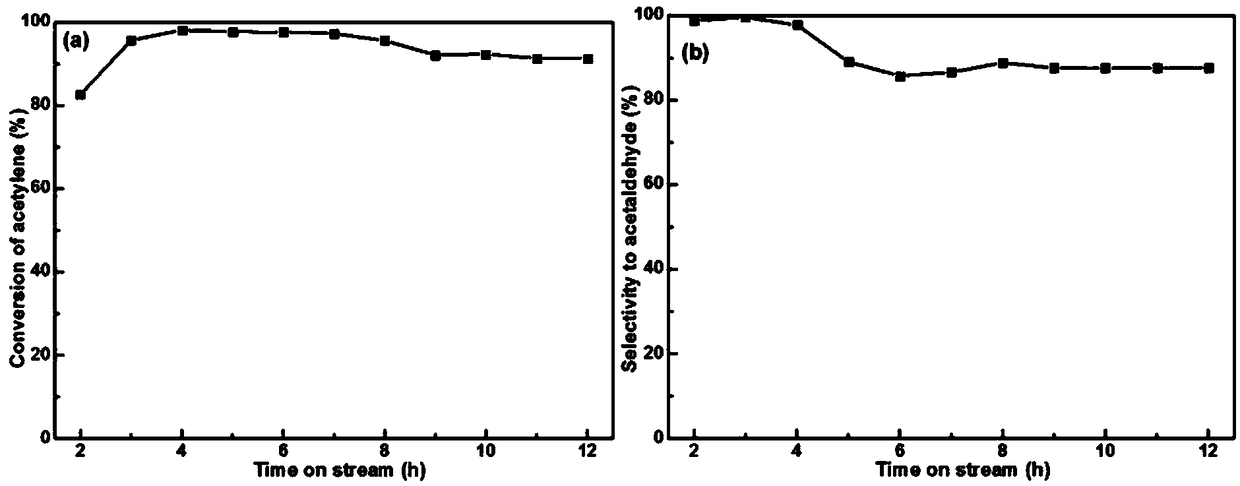
Zinc catalyst for catalyzing acetylene hydration reaction and preparation method of zinc catalyst
A zinc catalyst and acetylene hydration technology, which is applied in the preparation of carbon-carbon triple bond hydration, physical/chemical process catalysts, molecular sieve catalysts, etc., can solve the problems of high energy consumption and low selectivity, and achieve small particles and high selectivity , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The specific operation steps are as follows:
[0038] (1) Preparation of molecular sieve precursor solution: weigh 5.2 g of cetyltrimethylammonium bromide and 2.4 g of sodium hydroxide, and completely dissolve them in 200.0 mL of deionized water, mix and heat to 35 ° C after complete dissolution, Add 19.8 g of ethyl orthosilicate, and continue stirring for 1 h to obtain a molecular sieve precursor solution.
[0039] (2) Preparation of modified carrier: under vigorous stirring, 8 g of n-butyl titanate was added dropwise to the molecular sieve precursor solution, and after uniform stirring, a modified molecular sieve precursor solution was obtained.
[0040] The modified molecular sieve precursor solution was heated, stirred for 4 hours, then washed with water for several times until the pH value was neutral, dried at 60°C for 12 hours, calcined in a nitrogen tube furnace at 500°C for 5 hours, and then calcined in a muffle furnace at 500°C 6h, the modified molecular siev...
Embodiment 2
[0050] The operating steps of Example 2 are the same as those of Example 1, except that the amount of n-butyl titanate added is 1 g.
[0051] During the activity test, when the reaction temperature is 100-400°C, C 2 h 2 The flow rate is 3.4ml / min, H 2 The flow rate of O is 0.01g / min, and the space velocity is adjusted to 90h -1 Under certain conditions, the conversion rate of acetylene reaches the highest value of 78%, and the selectivity of acetaldehyde is greater than 75%.
Embodiment 3
[0053] The operation steps of Example 3 are the same as those of Example 1, except that the amount of n-butyl titanate added is 1.34 g.
[0054] During the activity test, when the reaction temperature is 100-400°C, C 2 h 2 The flow rate is 3.4ml / min, H 2 The flow rate of O is 0.01g / min, and the space velocity is adjusted to 90h -1 Under certain conditions, the conversion rate of acetylene reaches the highest value of 75%, and the selectivity of acetaldehyde is greater than 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com