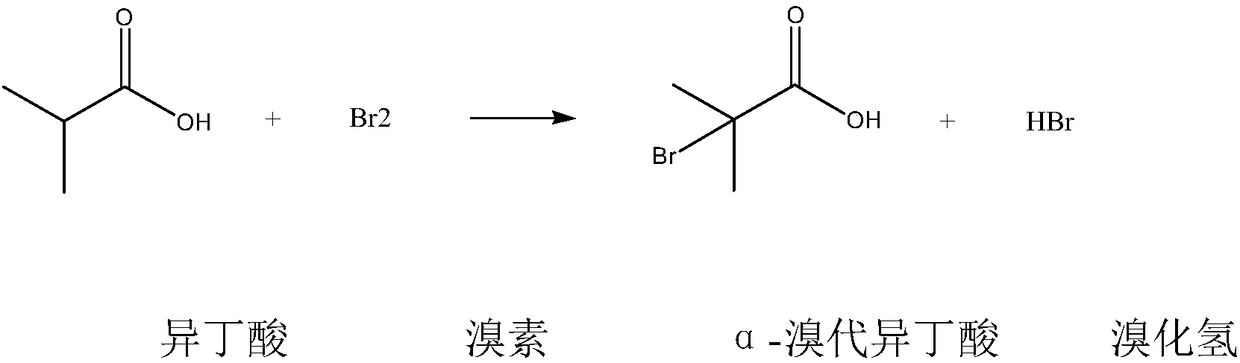
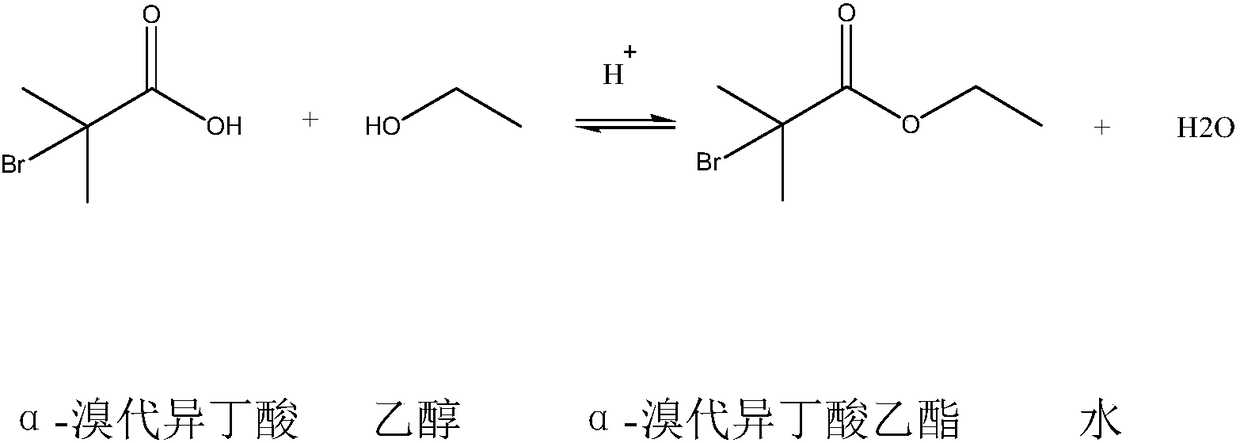
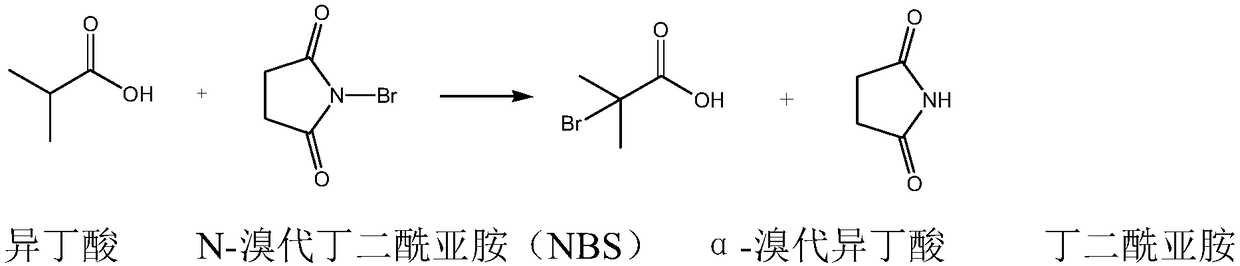
Method for synthesizing ethyl alpha-bromoisobutyrate
A technology of ethyl bromoisobutyrate and bromoisobutyric acid, which is applied in the field of synthesis of fine chemical intermediate α-ethyl bromoisobutyrate, can solve the problem of high risk, strong corrosion and inability to use bromine Solve problems such as high quality, achieve the effect of improving utilization rate, improving safety, and good appearance quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]In a 500ml four-necked flask with a stirring blade and a thermometer, successively put isobutyric acid: 35.5g (content: 99.0%, 0.3994mol), cyclohexane: 300ml (about 230g), dibenzoyl peroxide 0.25g, N-bromosuccinimide: 75.6 g (content: 98.8%, 0.4196 mol). Fix the water separator on the four-neck flask, and fix the condenser on the upper part of the water separator. Turn on the stirring and set the stirring speed above 500r / min. The outer wall of the four-necked bottle is heated with a water bath, and the temperature of the hot water in the water bath is set at 90°C. When the internal temperature of the four-necked bottle reaches about 82°C, the inside of the reaction bottle starts to boil and vaporize, and the vaporized cyclohexane dissolves a small amount of water in the reaction system. Take out the reaction vial and settle to the bottom of the trap.
[0045] As the reaction proceeds, the reaction feed liquid gradually changes from colorless to light yellow, and the N...
Embodiment 2
[0051] In a 500ml four-necked flask with a stirring blade and a thermometer, successively put isobutyric acid: 35.0g (content: 99.0%, 0.3938mol), cyclohexane: 380ml (about 300g), dibenzoyl peroxide 0.24g, N-bromosuccinimide: 75.7 g (content: 98.8%, 0.4202 mol). Fix the water separator on the four-neck flask, and fix the condenser on the upper part of the water separator. Turn on the stirring and set the stirring speed above 500r / min. The outer wall of the four-necked bottle is heated with a water bath, and the temperature of the hot water in the water bath is set at 90°C. When the internal temperature of the four-necked bottle reaches about 82°C, the inside of the reaction bottle starts to boil and vaporize, and the vaporized cyclohexane dissolves a small amount of water in the reaction system. Take out the reaction vial and settle to the bottom of the trap.
[0052] As the reaction proceeds, the reaction feed liquid gradually changes from colorless to light yellow, and the ...
Embodiment 3
[0058] In a 500ml four-necked flask with a stirring blade and a thermometer, successively put isobutyric acid: 35.0g (content: 99.0%, 0.3938mol), benzene: 380ml (about 330g), dibenzoyl peroxide 0.25g, N- Bromosuccinimide: 76.0 g (content: 98.8%, 0.4218 mol). Fix the water separator on the four-neck flask, and fix the condenser on the upper part of the water separator. Turn on the stirring and set the stirring speed above 500r / min. The outer wall of the four-necked bottle is heated with a water bath, and the temperature of the hot water in the water bath is set at 90°C. When the internal temperature of the four-necked bottle reaches about 82°C, the inside of the reaction bottle starts to boil and vaporize, and the vaporized cyclohexane dissolves a small amount of water in the reaction system. Take out the reaction vial and settle to the bottom of the trap.
[0059] As the reaction proceeds, the reaction feed liquid gradually changes from colorless to light yellow, and the N-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com