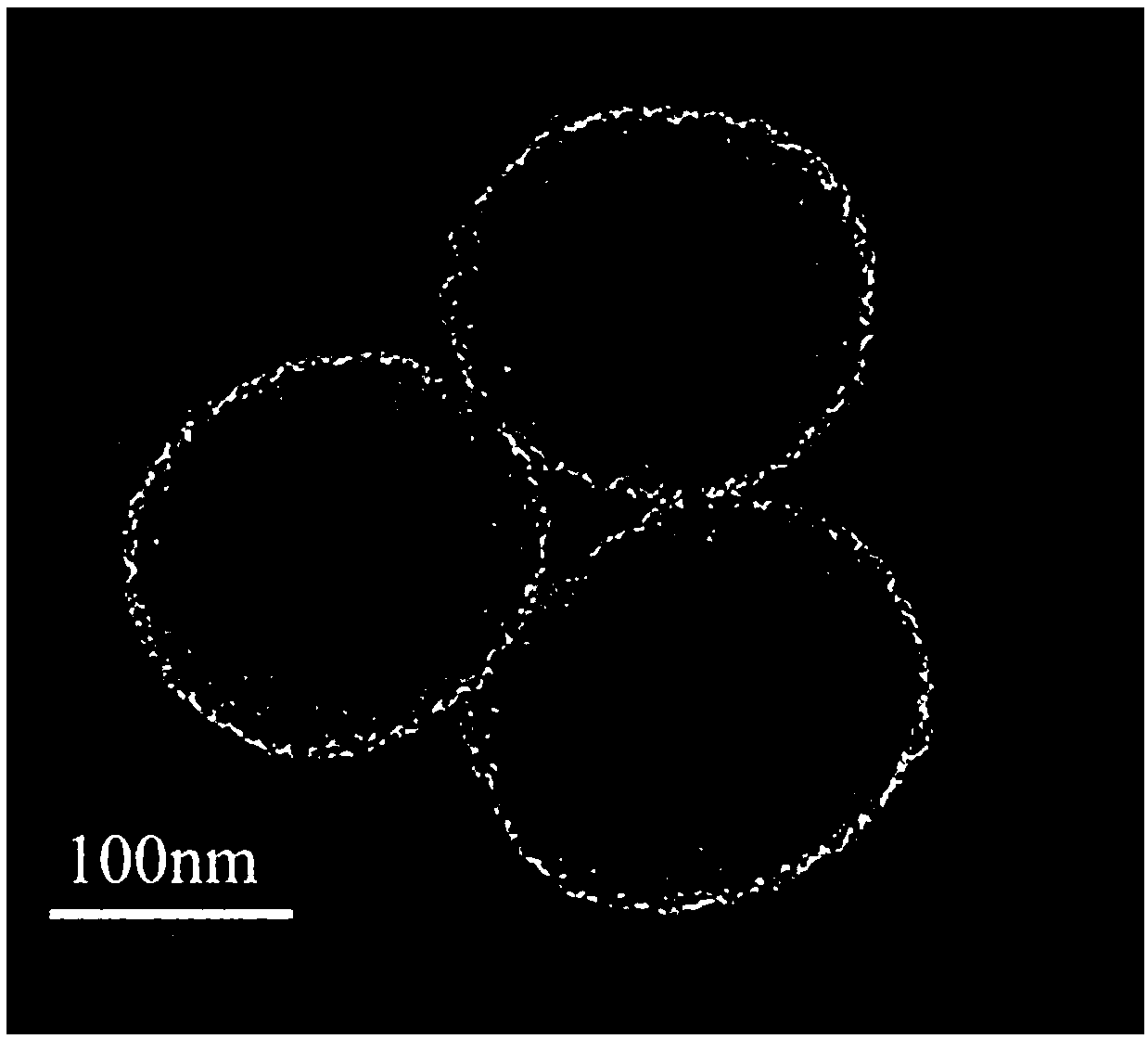
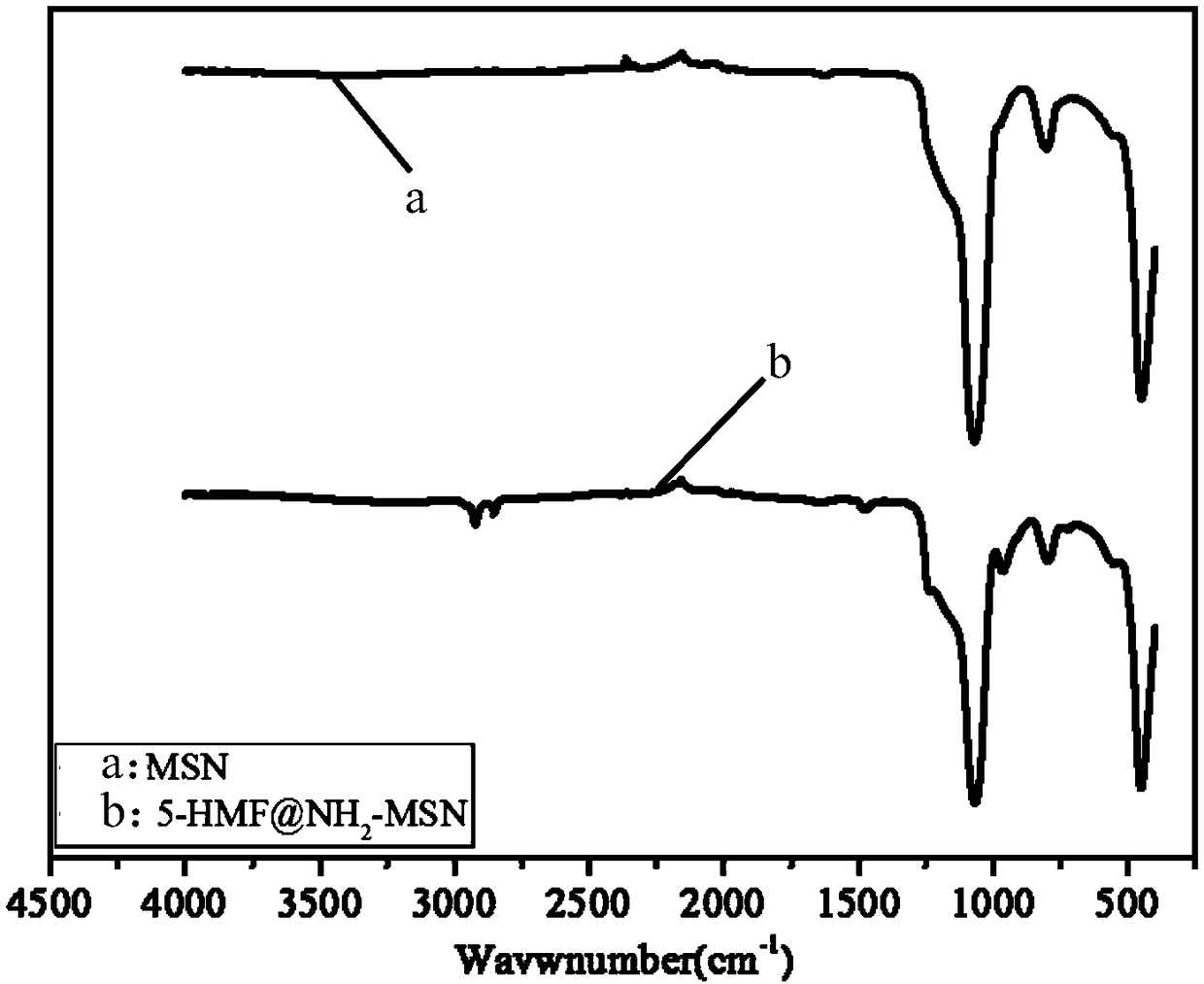
Method for preparing PH response amino-modified three-dimensional dendritic silica nanoparticle carrier carrying hydrophilic small molecule drug
An amino-modified, silicon dioxide technology, applied in pharmaceutical formulations, drug combinations, drug delivery, etc., can solve problems such as adverse reactions, and achieve the effects of preventing drug leakage, reducing pressure, and degrading quickly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Add 20 ml of CTAC solution and 0.2 g of TEA to 40 ml of deionized water, and gently stir at 60 °C for 1 h in a 100 mL round bottom flask;
[0030] (2) Then add 15 ml of 30 v / v % TEOS in 1-octadecene solution to the water-CTAC-TEA solution and keep it in an oil bath at 60 °C under magnetic stirring. The stirring rate was set at ~150 rpm, and then the reactant was kept at a constant temperature and stirred continuously for 24 h to obtain the first-dimensional product;
[0031] (3) Then, the upper phase 1-octadecene solution was completely removed and replaced with 20 v / v% TEOS in decahydronaphthalene to maintain the same reaction conditions for second-dimensional growth for another 24 h;
[0032] (4) For the third dimension, change the upper oil layer to 20 v / v% TEOS-cyclohexane solution, while keeping the reaction conditions under the same conditions for 24 h;
[0033] (5) Mix the carrier with 5-HMF at a ratio of 0.5:1 (m / m), disperse the carrier evenly by ultrasoun...
Embodiment 2
[0036] (1) Add 30ml of CTAC solution and 0.15g of TEA to 35ml of deionized water, and gently stir at 60°C for 1.5h in a 100mL round bottom flask;
[0037] (2) Then add 25ml of 30 v / v % TEOS in 1-octadecene solution to the water-CTAC-TEA solution and keep it in an oil bath at 60 °C under magnetic stirring. The stirring rate was set at ~150 rpm, and then the reactant was kept at a constant temperature and stirred continuously for 24 h to obtain the first-dimensional product;
[0038] (3) Then, the upper phase 1-octadecene solution was completely removed and replaced with 20 v / v% TEOS in decahydronaphthalene to maintain the same reaction conditions for second-dimensional growth for another 12 h;
[0039](4) For the third dimension, change the upper oil layer to 20 v / v% TEOS-cyclohexane solution, while keeping the reaction conditions at the same condition for 12 h;
[0040] (5) Mix the carrier and curcumin at a ratio of 0.75:1 (m / m), disperse the carrier evenly by ultrasound, the...
Embodiment 3
[0042] (1) Add 40 ml of CTAC solution and 0.1 g of TEA to 30 ml of deionized water, and gently stir at 60 °C for 2 h in a 100 mL round bottom flask;
[0043] (2) Then add 30 ml of 15 v / v % TEOS in 1-octadecene solution to the water-CTAC-TEA solution and keep it in an oil bath at 60 °C under magnetic stirring. The stirring rate was set at ~150 rpm, and then the reactant was kept at a constant temperature and stirred continuously for 24 h to obtain the first-dimensional product;
[0044] (3) Then, the upper phase 1-octadecene solution was completely removed and replaced with 20 v / v% TEOS in decahydronaphthalene to maintain the same reaction conditions for second-dimensional growth for another 48 h;
[0045] (4) For the third dimension, change the upper oil layer to 20 v / v% TEOS-cyclohexane solution, while keeping the reaction conditions at the same condition for 48 h;
[0046] (5) Mix the carrier and DOX at a ratio of 1:1 (m / m), disperse the carrier evenly by ultrasound, then r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap