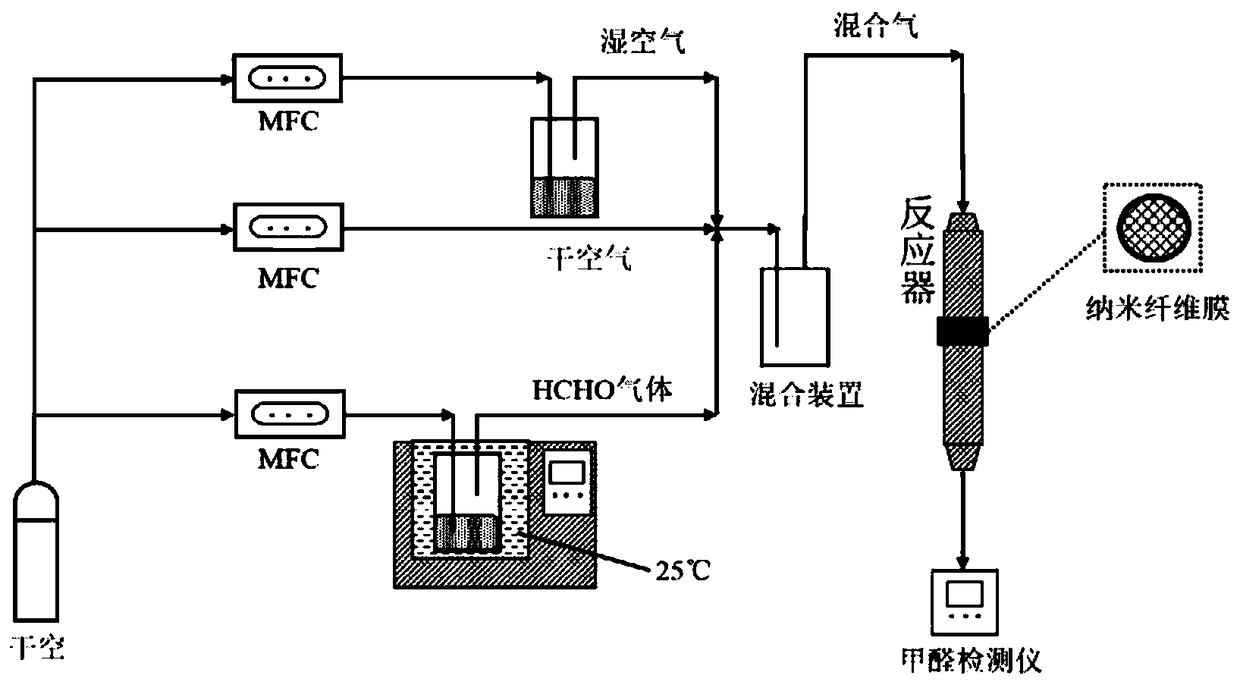
Nano-fiber membrane material capable of decomposing formaldehyde at normal temperature, and preparation method and application of same
A nanofiber membrane and formaldehyde technology, which is applied in separation methods, chemical instruments and methods, and dispersed particle separation, etc., can solve problems such as catalyst loss, catalyst agglomeration, and large air resistance, and achieve long service life, high catalytic performance, and satisfaction The effect of industry requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Weigh 1.975gCo(NO 3 ) 2 ·6H 2 O, 1.704gMn(NO 3 ) 2 4H 2 O and 0.417g of isonicotinic acid were placed in a stainless steel ball milling jar, four stainless steel ball milling beads with a diameter of 10mm were added, put into a high-speed vibrating ball mill, and ball milled at 1000r / min for 20min to obtain a solid powder. The powder was soaked in DMF solution at 50°C for 24h, the solution was changed every 6h, centrifuged at 10000r / min for 5min, and the lower layer product was vacuum-dried at 80°C for 24h to obtain Mn / Co-MOFs.
[0046] (2) The MOFs material obtained above was put into a muffle furnace and calcined at 350°C for 2.5h to prepare the Mn-Co-1 catalyst.
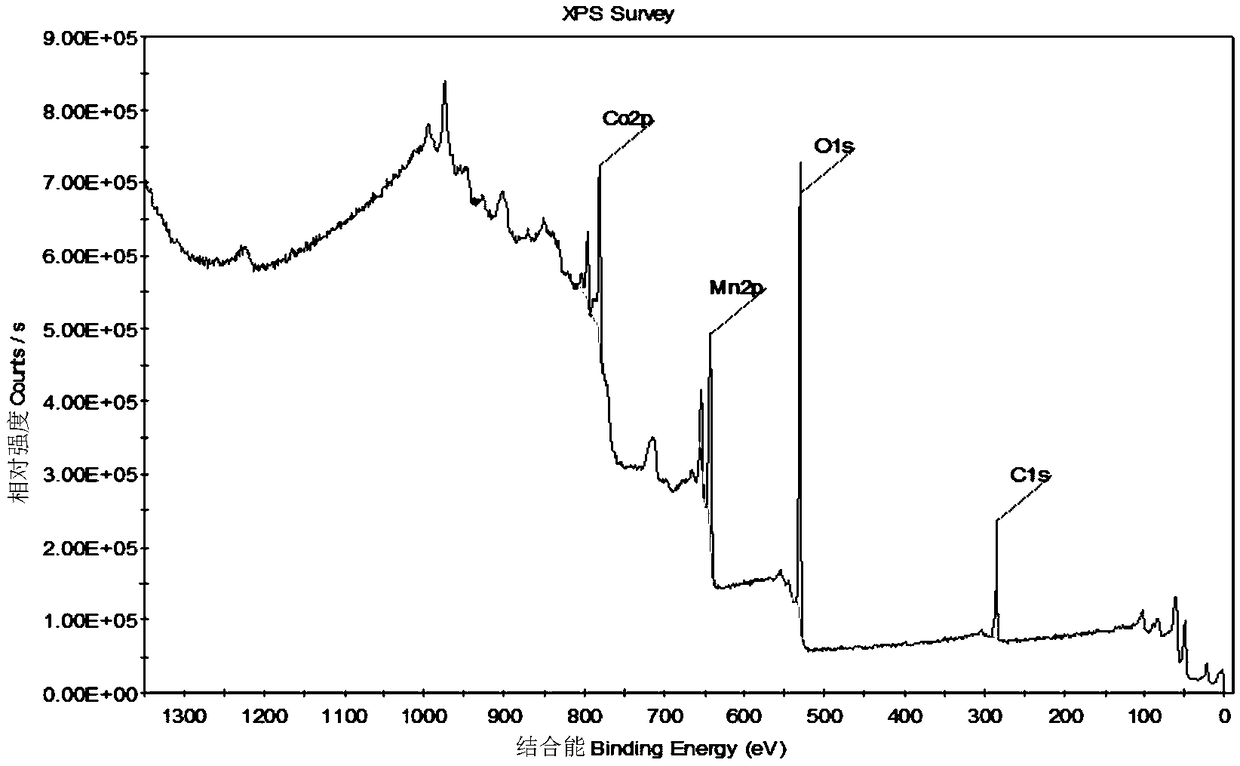
[0047] Carry out XPS analysis to the catalyst synthesized by the present embodiment, its result is as follows figure 1 As shown, it can be seen from the figure that the catalyst contains Mn, Co, O, and C elements, which proves that Mn, Co elements are successfully embedded in the catalyst, C is a ...
Embodiment 2
[0049] (1) Weigh 0.881g CoCO 3 , 1.704gMnCO 3 and 1.334g of oxalic acid were placed in a stainless steel ball milling jar, four stainless steel ball milling beads with a diameter of 9mm were added, put into a high-speed vibrating ball mill, and ball milled for 20min at a speed of 1000r / min to obtain a solid powder. The powder was soaked in DMF solution at 50°C for 24h, the solution was changed every 6h, centrifuged at 10000r / min for 5min, and the lower layer product was vacuum-dried at 80°C for 24h to obtain Mn / Co-MOFs.
[0050] (2) Put the MOFs material obtained above into a muffle furnace for 2.5 h at 350° C. to prepare the Mn-Co-2 catalyst. The XPS spectrum and figure 1 Similarly, it shows that the catalyst contains Mn, Co, O, and C elements. It proves that Mn, Co elements are successfully embedded in the catalyst, and C is a small amount. The modified carbonization temperature is not enough for metal ions to react with C. Therefore, it is proved that Mn, Co, O is based o...
Embodiment 3
[0052] (1) Weigh 4.294gCoSO 4 ·7H 2 O, 1.704gMnSO 4 4H 2 0 and 3.210g of trimesic acid were placed in a stainless steel ball milling jar, four stainless steel ball milling beads with a diameter of 8mm were added, put into a high-speed vibrating ball mill, and ball milled for 20min at a speed of 1000r / min to obtain a solid powder. The powder was soaked in DMF solution at 50°C for 24h, the solution was changed every 6h, centrifuged at 10000r / min for 5min, and the lower layer product was vacuum-dried at 80°C for 24h to obtain Mn / Co-MOFs.
[0053] (2) Put the MOFs material obtained above into a muffle furnace for 2.5 h at 350 ° C to prepare the Mn-Co-3 catalyst, the XPS spectrum and figure 1 Similarly, it shows that the catalyst contains Mn, Co, O, and C elements. It proves that Mn, Co elements are successfully embedded in the catalyst, and C is a small amount. The modified carbonization temperature is not enough for metal ions to react with C. Therefore, it is proved that Mn, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com