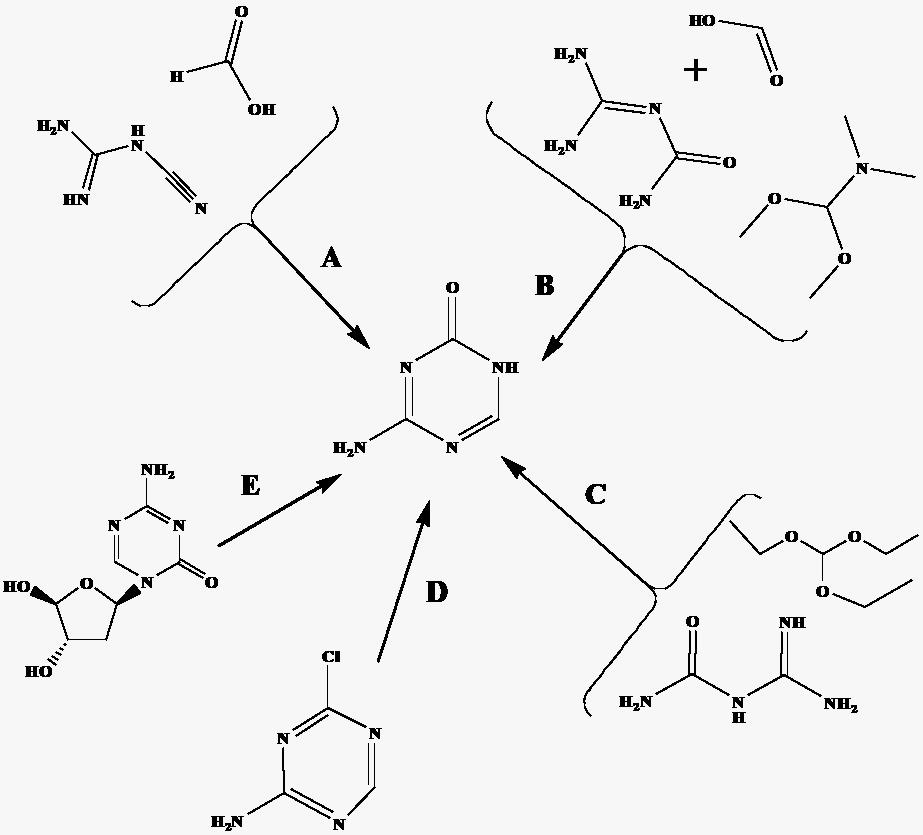
A new method for the synthesis of 5-azacytosine
A technique for the synthesis of azacytosine, which is applied in the field of organic synthesis, can solve the problems of complicated reaction temperature product purification process and low process yield, and achieve the effects of easy biochemical degradation treatment, simple process, and improved conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Weigh 42 g (0.5mol) of dicyandiamide and place it in a dry reactor, measure 76 mL (1.25mol) of methyl formate into the reactor and mix with dicyandiamide, put the reactor into The temperature in the oven was raised to 115°C, kept warm for 120 minutes, then taken out, the liquid in the kettle was evaporated, cooled, and the white solid produced was taken out.
[0044] (2) Transfer the white solid to a 1000 mL three-necked flask, and add 200 mL of 10% dilute hydrochloric acid. Heat to reflux for 30 minutes until the solution becomes clear, filter the clear solution while it is hot, add ammonia water to the filtrate to adjust the pH to 6, and white crystals are precipitated, after centrifugation, dry at 120°C in a hot air oven to obtain 40 g of 5-azacytosine ( The yield is 71% based on dicyandiamide).
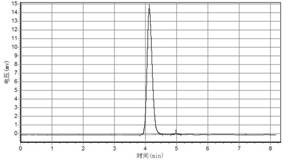
[0045] Depend on image 3 Infrared spectroscopy can confirm that the synthetic product is 5-azacytosine; Figure 4 It is the high-performance liquid chromatogram of ...
Embodiment 2
[0047] (1) Weigh 42 g (0.5mol) of dicyandiamide and place it in a dry reactor, measure 100 mL (1.25mol) of ethyl formate into the reactor and mix with dicyandiamide, put the reactor into The temperature in the oven was raised to 115°C, kept warm for 120 minutes, then taken out, the liquid in the kettle was evaporated, cooled, and the white solid produced was taken out.
[0048] (2) Transfer the white solid to a 1000 mL three-necked flask, and add 200 mL of 10% dilute hydrochloric acid. Heat to reflux for 30 minutes until the solution becomes clear, filter the clear solution while it is hot, add ammonia water to the filtrate to adjust the pH to 6, and white crystals are precipitated, after centrifugation, dry at 120°C in a hot air oven to obtain 42 g of 5-azacytosine ( The yield is 75% based on dicyandiamide).
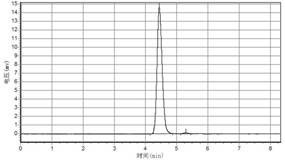
[0049] Depend on Figure 5 Draw, utilize area normalization method to test purity higher than 98% by high performance liquid chromatography.
Embodiment 3
[0051] (1) Weigh 42 g (0.5mol) of dicyandiamide and place it in a dry reactor, measure 120 mL (1.25mol) of propyl formate into the reactor and mix with dicyandiamide, put the reactor into The temperature in the oven was raised to 115°C, kept warm for 120 minutes, then taken out, the liquid in the kettle was evaporated, cooled, and the white solid produced was taken out.
[0052] (2) Transfer the white solid to a 1000 mL three-necked flask, and add 200 mL of 10% dilute hydrochloric acid. Heat to reflux for 30 minutes until the solution becomes clear, filter the clear solution while it is hot, add ammonia water to the filtrate to adjust the pH to 6, and white crystals are precipitated, after centrifugation, dry in a hot air oven at 120°C to obtain 45g of 5-azacytosine (The yield is 80% based on dicyandiamide).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com