Method for preparing ziconotide by solid-liquid combination
A ziconotide, solid-liquid phase technology, applied in the field of peptide compound synthesis, can solve the problems of long synthesis cycle, low product yield, unfavorable industrialization amplification, and high production cost, and achieve easy operation, shorten synthesis cycle, improve purity and Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Boc-Cys(trt)-Lys(boc)-Gly-Lys(boc)-Gly-Ala-Lys(boc)-Cys(trt)-Ser(tbu)-Arg(pbf)-Leu-Met - Preparation of Tyr(tbu)-Asp(otbu)-COOH;
[0054] Weigh 20g (0.3mmol / g) of Fmoc-Asp(otbu)-2Cl-Trt-Cl-resin and place it in the reaction column, add 150ml of 20% piperidine / DMF solution by volume percentage, react for 30 minutes, and drain. Wash with DMF for 5 times, then weigh the protected amino acid and condensing agent, add to the reaction column, then add 100ml of DMF, react for 30-60 minutes, detect the end of the reaction with ninhydrin, drain the reaction solution after the reaction, wash with DMF for 3 times, Repeat the cycle until the last amino acid is connected to get Boc-Cys(trt)-Lys(boc)-Gly-Lys(boc)-Gly-Ala-Lys(boc)-Cys(trt)-Ser(tbu)-Arg (pbf)-Leu-Met-Tyr(tbu)-Asp(otbu)-2Cl-Trt-Cl-resin 36g.
[0055] The amount of amino acid added in each step of condensation reaction is respectively:
[0056] Fmoc-Tyr(tbu)-OH 5.52g,
[0057] Fmoc-Met-OH 4.46g,
[0058] ...
Embodiment 2
[0071] Embodiment 2: Preparation of Cys-Lys-Gly-Lys-Gly-Ala-Lys-Cys-Ser-Arg-Leu-Met-Tyr-Asp-Sbzl;
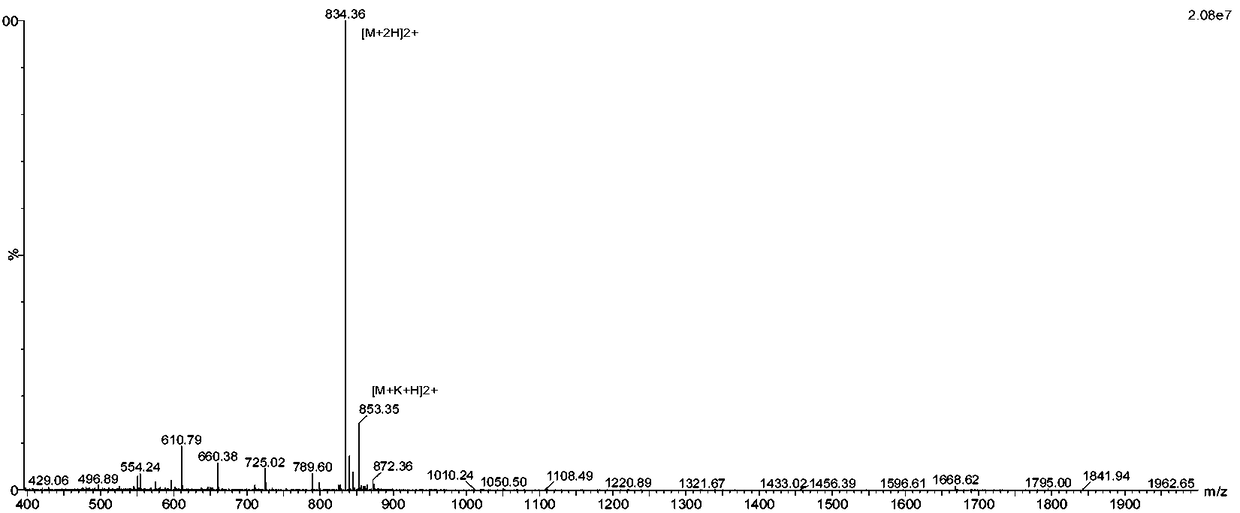
[0072] Get 4.2g of fully protected polypeptide fragment A in Example 1 and place it in a flask, add 100ml DMF to dissolve, then add 0.37g benzyl mercaptan, 1.24g HCTU, 5ml DIEA, react for 1 hour, add water to separate out a white solid, filter to remove the filtrate , after the filter cake is dried, add 200ml of secondary lysate, the ratio (V%) consists of: 87.5% TFA + 5% sulfide anisole + 2.5% ethanedithiol + 2.5% phenol + 2.5% water, the reaction After 2 hours, 800ml of ether was added to crystallize, and a white solid was precipitated. After drying, the polypeptide fragment A was obtained: Cys-Lys-Gly-Lys-Gly-Ala-Lys-Cys-Ser-Arg-Leu-Met-Tyr-Asp-Sbzl 2.25 g, MW: 1666, see product mass spectrum figure 1 .
Embodiment 3
[0073] Embodiment 3: Preparation of Cys-Lys-Gly-Lys-Gly-Ala-Lys-Cys-Ser-Arg-Leu-Met-Tyr-Asp-Sbzl;
[0074] Get 4.2g of fully protected polypeptide fragment A in Example 1 and place it in a flask, add 100ml NMP to dissolve, then add 0.37g benzyl mercaptan, 1.24g HCTU, 5ml DIEA, react for 2 hours, add water to separate out a white solid, filter to remove the filtrate , after the filter cake is dried, add 200ml of secondary lysate, the ratio (V%) consists of: 87.5% TFA + 5% sulfide anisole + 2.5% ethanedithiol + 2.5% phenol + 2.5% water, the reaction After 2 hours, 800ml of ether was added to crystallize, and a white solid was precipitated. After drying, the polypeptide fragment A was obtained: Cys-Lys-Gly-Lys-Gly-Ala-Lys-Cys-Ser-Arg-Leu-Met-Tyr-Asp-Sbzl 2.35 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com