Polycarbonyl-substituted chiral pyrrolidine derivative and preparation method thereof
A technology of derivatives and pyrrolidine, which is applied in the field of polycarbonyl functionally substituted chiral pyrrolidine derivatives and its preparation, can solve the problem of high enantioselectivity and few synthetic methods of polycarbonyl functionally substituted chiral pyrrolidine derivatives, etc. problem, to achieve the effect of high enantioselectivity, considerable application value, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
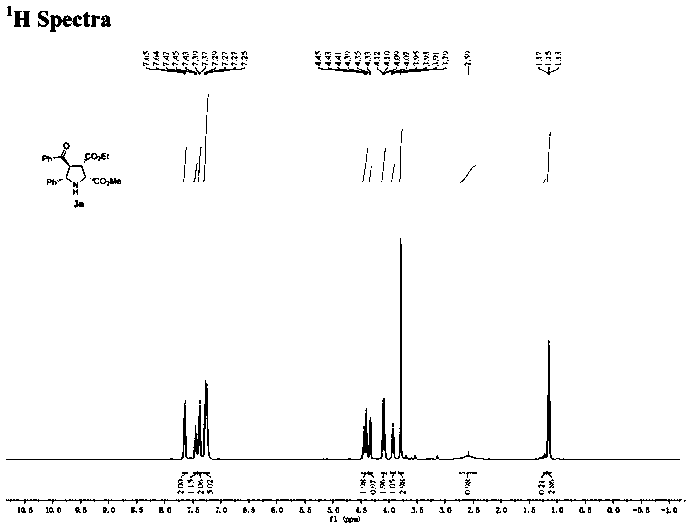
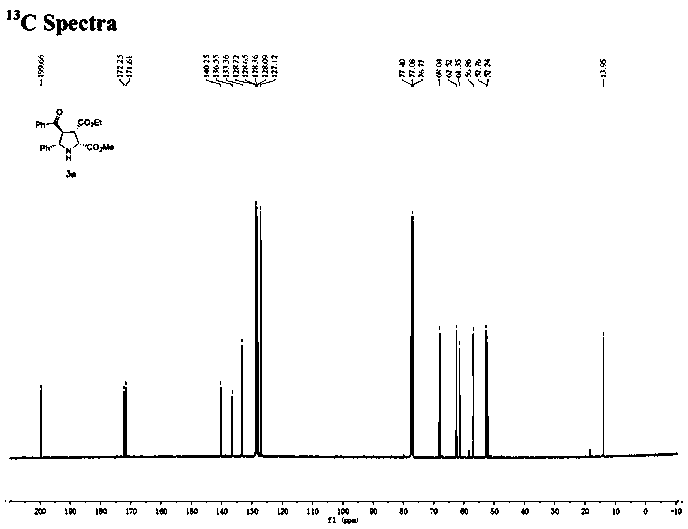
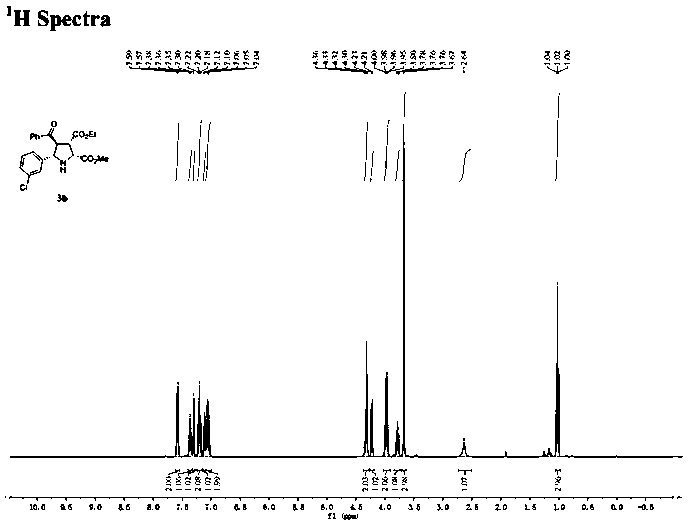
[0030] Under nitrogen atmosphere, (R)-DTBM-SegPhos (13mg, 0.0108mmol, 6mol%), AgOAc (1.6mg, 0.0096mmol, 5mol%) was added to the dissolved chalcone (3.8mg, 0.0182mmol, 10mol%) ) in 2 mL of acetone solution, after pre-stirring for 30 minutes at -40°C, add methyleneamine ylide 1a (0.2mmol) and enone compound 2a (0.182mmol) in sequence, react at -40°C for 12h, and monitor the reaction by TLC , filtered through diatomaceous earth, extracted, the filtrate was concentrated, and purified by silica gel column chromatography to obtain a colorless oily liquid 3a with a yield of 68%. The ee value was measured by chiral high performance liquid chromatography to be 96%.
[0031]
[0032] The physicochemical index of this product: 1 H NMR (400MHz, CDCl 3 )δ7.65(d, J=7.4Hz, 2H), 7.45(t, J=7.4Hz, 1H), 7.38(d, J=6.7Hz, 2H), 7.27(dd, J=11.3, 7.7Hz, 5H), 4.42(dd, J=17.5, 8.1Hz, 2H), 4.34(d, J=8.4Hz, 1H), 4.09(q, J=7.1Hz, 2H), 3.93(t, J=7.9Hz, 1H), 3.79(s, 3H), 2.59(s, 1H), 1.15(t, J=7.1Hz...
Embodiment 2
[0034] The preparation method was the same as in Example 1, and the dissolved chalcone was changed to 8mol% (3.04mg, 0.0146mmol) to obtain a colorless oily liquid 3a with a yield of 58%, and the ee value measured by chiral high-performance liquid chromatography was 95%. .
Embodiment 3
[0036]The preparation method was the same as in Example 1, and the dissolved chalcone was changed to 6mol% (2.28mg, 0.0109mmol) to obtain a colorless oily liquid 3a with a yield of 63%, and the ee value measured by chiral high-performance liquid chromatography was 94%. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com