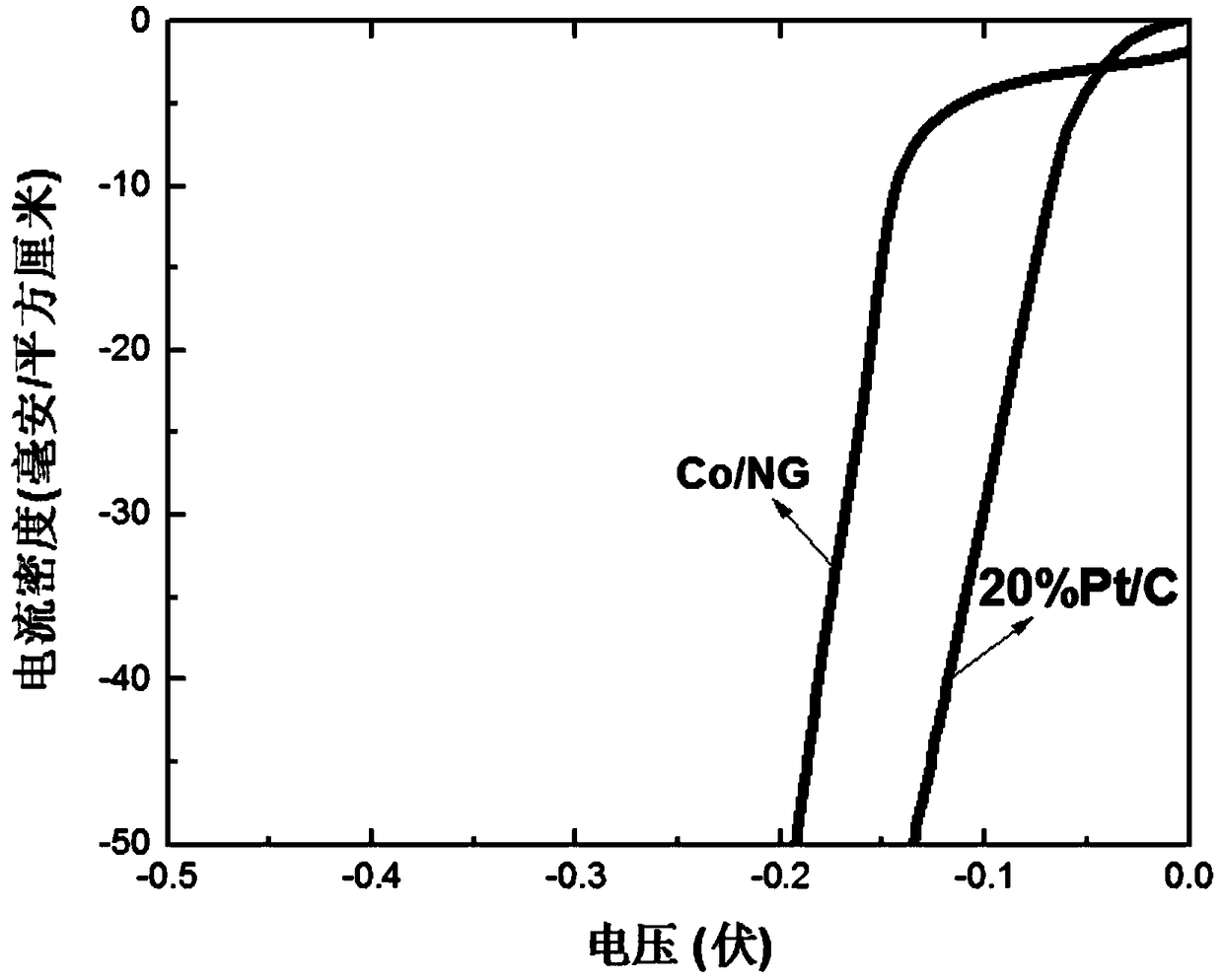
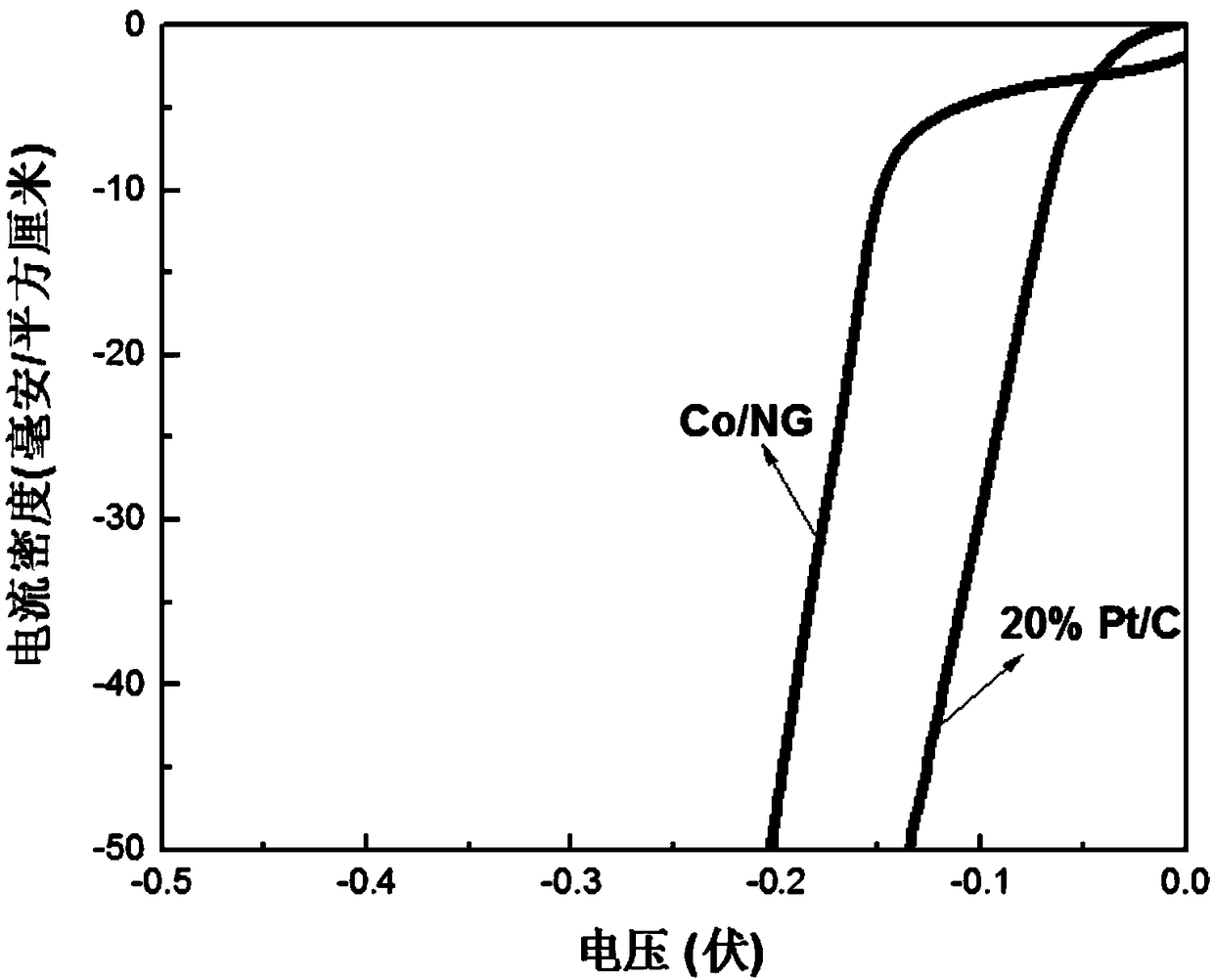
A preparation method of Co/N/C catalyst for hydrogen precipitation reaction
A catalyst and reaction technology, applied in the direction of electrical components, battery electrodes, circuits, etc., can solve environmental pollution and other problems, and achieve the effect of uniform distribution and good electrocatalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In this embodiment, the Co / N / C HER catalyst is realized according to the following steps:
[0029] (1) Configuration of solution (1): Weigh 0.6g of cobalt acetate tetrahydrate and dissolve it in 10g of N,N-dimethylformamide solution, then transfer the above mixed solution to a sealed beaker and place it under magnetic force Stir on the stirrer, the stirring rate is 500rpm, the stirring temperature is 40°C, and the liquid in the beaker is stirred to a uniform purple liquid, and the solution (1) is obtained.
[0030] (2) Configuration of solution (2): Dissolve 1.06g of polyacrylonitrile in the solution (1) prepared in step (1), then transfer the above mixed solution to a sealed beaker, and stir on a magnetic stirrer , the stirring rate is 500rpm, the stirring temperature is 45°C, and the liquid in the beaker is stirred until it becomes a uniform purple liquid, and then the solution (2) is prepared.
[0031] (3) Spinning: transfer the solution (2) prepared in step (2) to ...
Embodiment 2
[0035] In this embodiment, the Co / N / C HER catalyst is realized according to the following steps:
[0036] (1) Configuration of solution (1): Weigh 0.9 mg of cobalt acetate tetrahydrate and dissolve it in 10 g of N,N-dimethylformamide solution, then transfer the above mixed solution to a sealed beaker and place it in a magnetic Stir on the stirrer, the stirring rate is 600rpm, the stirring temperature is 45°C, and the liquid in the beaker is stirred until it becomes a uniform purple liquid, and then the solution (1) is prepared.
[0037] (2) Configuration of solution (2): Dissolve 1.417g of polyacrylonitrile in the solution (1) prepared in step (1), then transfer the above mixed solution to a sealed beaker, and stir on a magnetic stirrer , the stirring rate is 600rpm, the stirring temperature is 50°C, and the liquid in the beaker is stirred until it becomes a uniform purple liquid, and then the solution (2) is prepared.
[0038] (3) Spinning: transfer the solution (2) prepared...
Embodiment 3
[0042] In this embodiment, the Co / N / C HER catalyst is realized according to the following steps:
[0043] (1) Configuration of solution (1): Weigh 0.2g of cobalt acetate tetrahydrate and dissolve it in 10g of N,N-dimethylformamide solution, then transfer the above mixed solution to a sealed beaker and place it under magnetic force Stir on the stirrer, the stirring rate is 450rpm, the stirring temperature is 35°C, and the liquid in the beaker is stirred until it becomes a uniform purple liquid, and the solution (1) is obtained.
[0044] (2) Configuration of solution (2): Dissolve 0.816g of polyacrylonitrile in the solution (1) prepared in step (1), then transfer the above mixed solution to a sealed beaker, and stir on a magnetic stirrer , the stirring rate is 400rpm, the stirring temperature is 40°C, and the liquid in the beaker is stirred until it becomes a uniform purple liquid, and then the solution (2) is prepared.
[0045](3) Spinning: transfer the solution (2) prepared i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com