Multifunctional high molecular micelle drug delivering system and preparation method and application thereof
A technology of polymer micelles and delivery systems, applied in the field of design targets, to increase the variety, enhance stability, and facilitate research and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
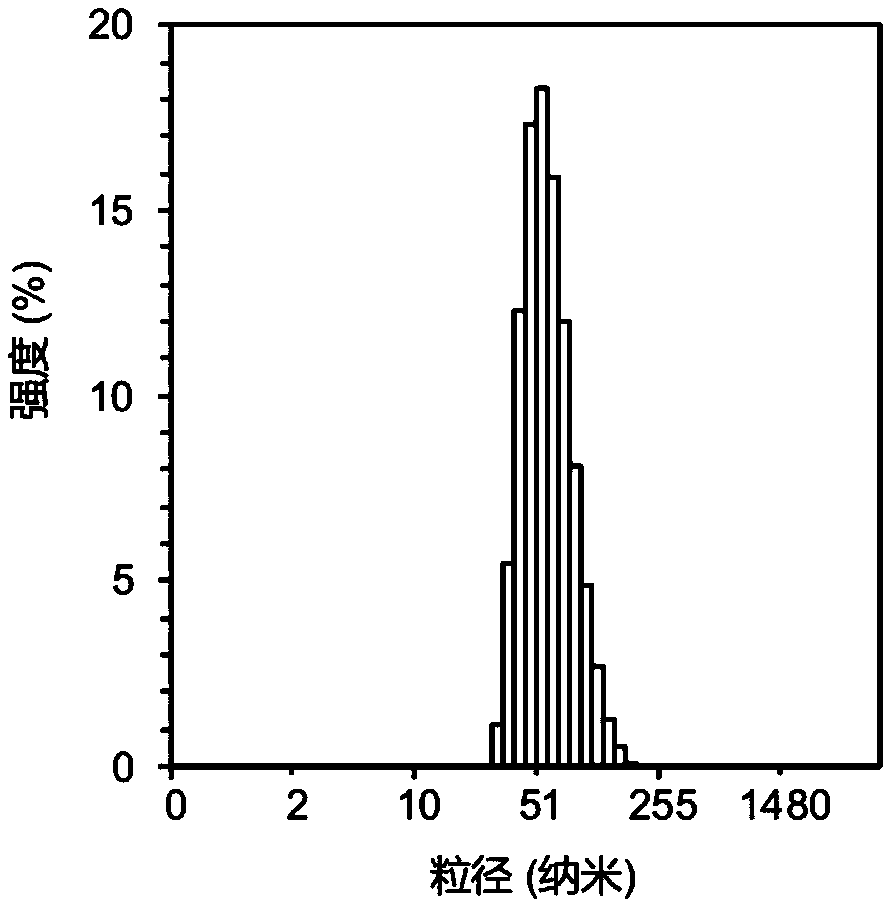
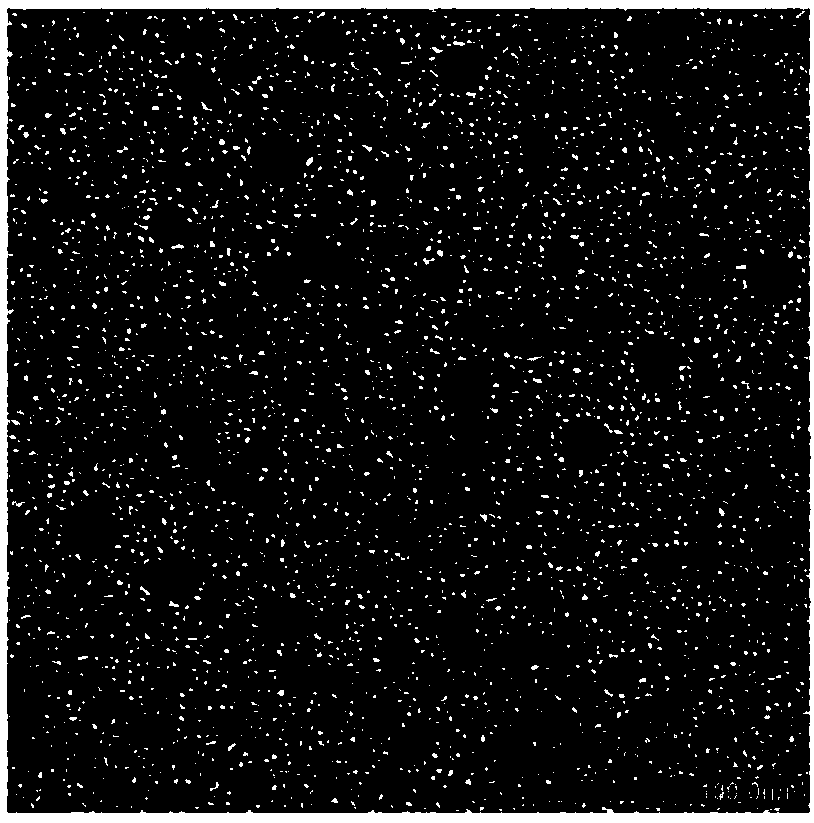
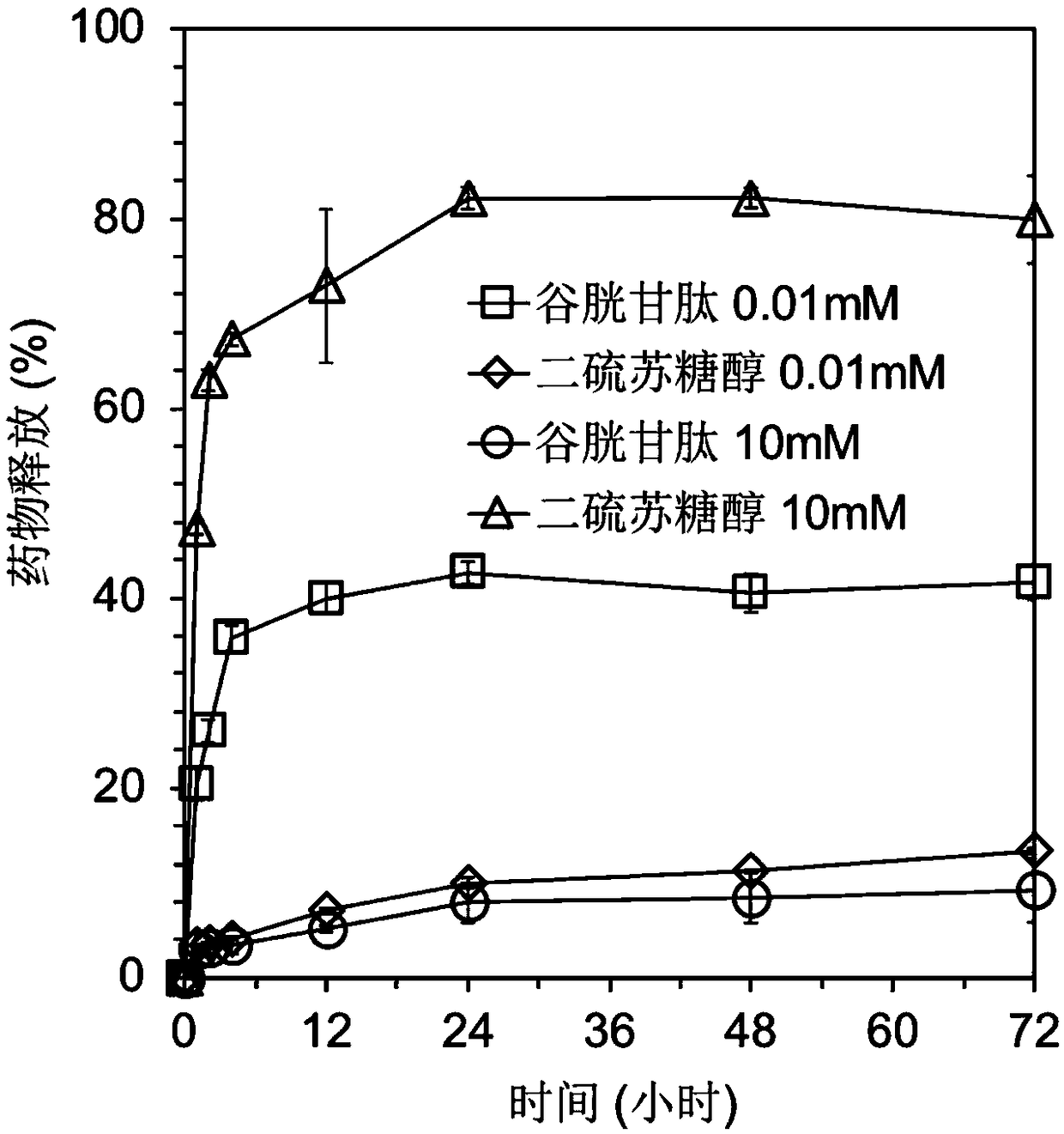
[0044] In this example, polyethylene glycol-polylysine block copolymer and 3,3'-dithiobispropioimidate dimethyl dihydrochloride were used as raw materials to construct a polymer micellar drug carrier system. The drug molecule is mercaptododecaborane disodium salt, and the process steps are as follows:
[0045] (1) Synthesis of polymer materials
[0046] Dissolve 50 mg of 3,3'-dithiobispropioimidate dimethyl dihydrochloride side chain modified polyethylene glycol-polylysine block copolymer in 15 ml of HEPES buffer solution (10 mM , pH 7.4), 34 mg of medicinal mercaptododecaborane disodium was dissolved in 4 ml of HEPES buffer solution (10 mM, pH 7.4), then the two solutions were mixed, stirred and reacted for 6 hours, and then the products were washed with HEPES respectively The buffer solution and pure water were dialyzed for 24 hours to form polymer micelles dissolved in disodium mercaptododecaborane through self-assembly.
[0047] (2) Cross-linking of drugs and polymer mat...
Embodiment 2
[0051] In this example, polyethylene glycol-polylysine block copolymer and 3,3'-dithiobispropioimidate dimethyl dihydrochloride were used as raw materials to construct a polymer micellar drug carrier system. The drug molecule is mercaptododecaborane disodium salt, and the process steps are as follows:
[0052] (1) Synthesis of polymer materials
[0053] At 25°C, 100 mg of polyethylene glycol-polylysine block copolymer was dissolved in 10 mL of borate buffer with a concentration of 100 mM and a pH value of 9.0, while 50 mg of 3,3'-bis Thiobispropionimidate dimethyl dihydrochloride was dissolved in 12mL concentration of 100mM, pH value of 9.0 borate buffer, the two solutions were mixed at room temperature for 60 minutes, and then the reaction product in 10mM, pH7 .4 Dithiothreitol was dialyzed three times, then added 70mg dithiothreitol to react for 50 minutes, then dialyzed three times with phosphate buffer solution and pure water, and finally obtained 3,3'-dithiobispropionimi...
Embodiment 3
[0057] In this example, polyethylene glycol-polylysine block copolymer and 3,3'-dithiobispropioimidate dimethyl dihydrochloride were used as raw materials to construct a polymer micellar drug carrier system. The drug molecule is mercaptododecaborane disodium salt, and the process steps are as follows:
[0058] (1) Synthesis of polymer materials
[0059] At 25°C, 100 mg of polyethylene glycol-polylysine block copolymer was dissolved in 10 mL of borate buffer with a concentration of 100 mM and a pH value of 9.0, while 150 mg of 3,3'-bis Thiobispropionimidate dimethyl dihydrochloride was dissolved in 15mL concentration of 100mM, pH value of 9.0 borate buffer, the two solutions were mixed at room temperature for 120 minutes, and then the reaction product in 10mM, pH7 .4 Dithiothreitol was dialyzed three times, then added 200 mg dithiothreitol to react for 60 minutes, then dialyzed three times with phosphate buffer solution and pure water, and finally obtained 3,3'-dithiobispropio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com