2,4-bis(diphenylphosphine oxide) tetrahydroquinoline compound and preparation method and application thereof
A technology of diphenylphosphine oxide and diphenylphosphine oxide, which is applied in the direction of phosphorus organic compounds, drug combinations, nervous system diseases, etc., can solve the problems of difficult synthesis and limited synthesis methods of phosphorus-nitrogen-containing heterocyclic compounds, and achieve structural Novel, non-toxic raw materials, good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] In order to describe the technical content, achieved goals and effects of the present invention in detail, the following descriptions will be made in conjunction with the embodiments and accompanying drawings.
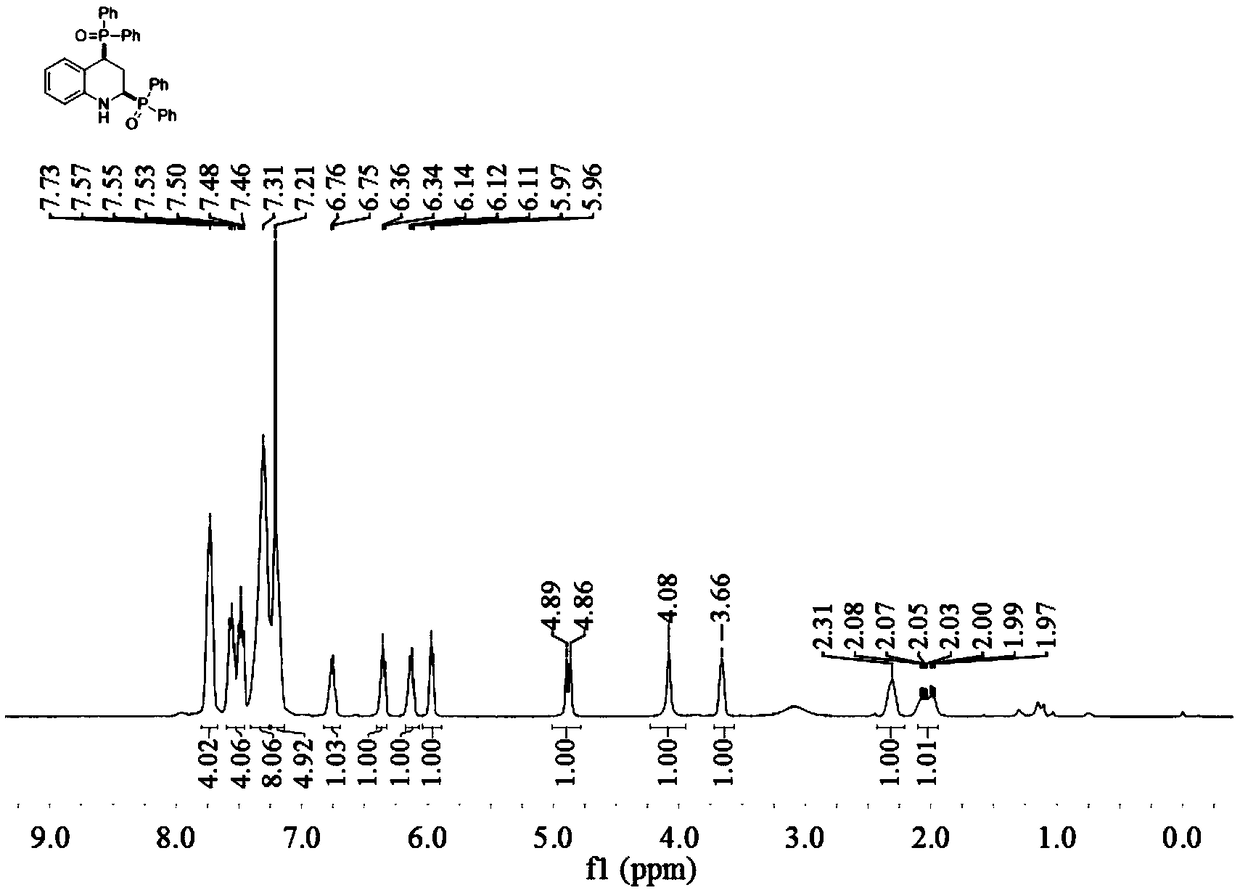
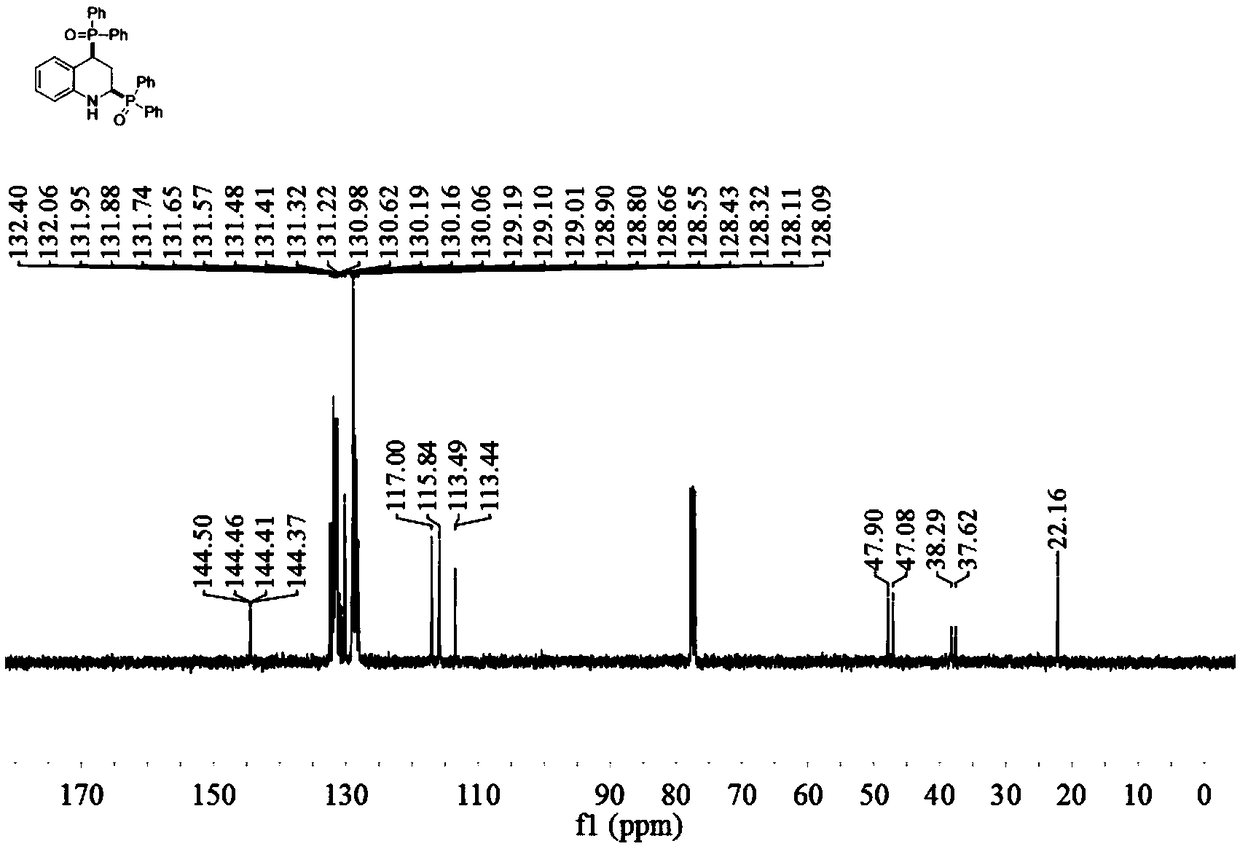
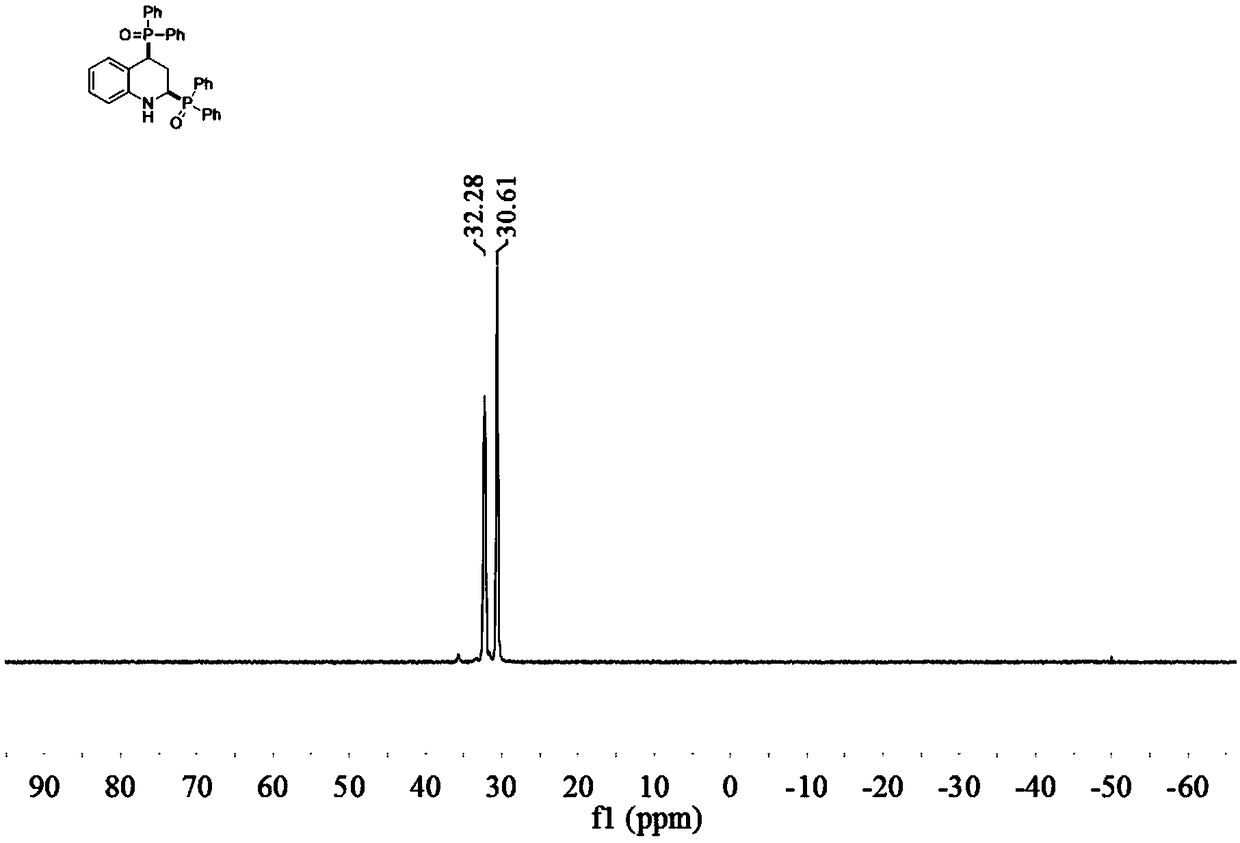
[0034] Embodiment 1 of the present invention is 2,4-bis(diphenylphosphine oxide)tetrahydroquinoline and its preparation method. The method comprises the following steps: in a reaction vessel, add 0.5 mmol quinoline and 1.0 mmol dihydroquinoline Phenylphosphine oxide, 0.01 mmol 1,5-cyclooctadiene iridium chloride dimer, 0.5 mmol isopropanol, 1.5 ml toluene, stirred and reacted at 100°C for 16 hours, cooled to room temperature after the reaction, Dilute the reaction solution, filter, and remove the solvent by rotary evaporation under reduced pressure to obtain the crude product, which is purified by column chromatography to obtain 2,4-bis(diphenylphosphine oxide)tetrahydroquinoline (3a) in the form of a yellow solid And the melting point is: 179.0-180.6°C.
[003...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com