Azo disperse dye compound, synthesis method thereof and application of compound
A technology of disperse dyes and compounds, applied in the direction of azo dyes, monoazo dyes, chemical instruments and methods, etc., can solve the problems of poor washing fastness and poor application performance, so as to improve affinity, dyeing performance and color Fastness, enhance the effect of van der Waals force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
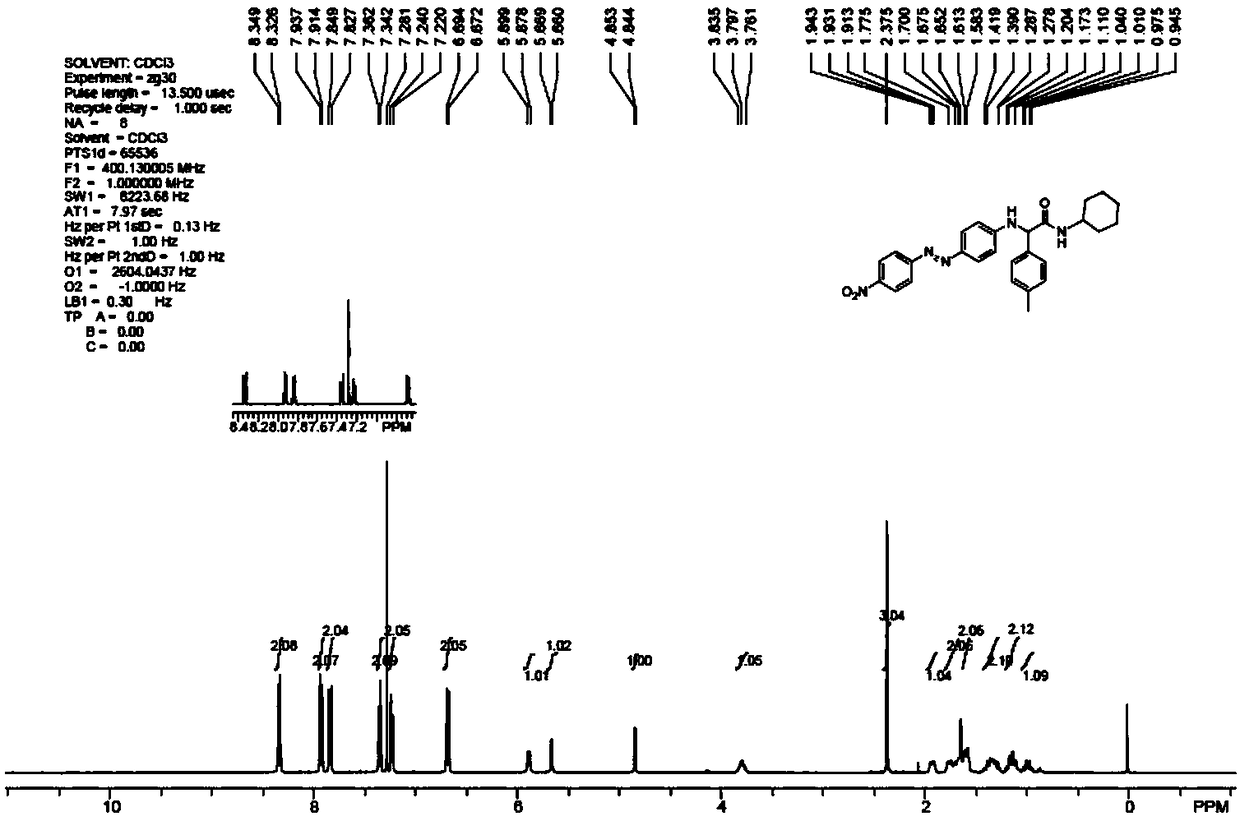
[0048] Example 1: Dye 1a
Embodiment 1-1
[0049] Embodiment 1-1: Preparation of Dye 1a
[0050] (1) Ugi four-component reaction:
[0051] In a 250mL round bottom flask, add anhydrous methanol (150mL), aniline (4.39mL, 48.16mmol, 1.05eq), p-tolualdehyde (4.9mL, 48.16mmol, 1.05eq), formic acid (2.07mL, 55.04mmol , 1.2eq), and cyclohexylacetonitrile (5.69mL, 45.87mmol, 1.0eq). The reaction mixture was heated and stirred in an oil bath at 80° C. for 48 h, and analyzed by TLC, the reaction was complete. After the reaction solution was cooled to room temperature, it was cooled in the lower layer of the refrigerator overnight to produce a white solid. Suction filtration (washing with EA:PE=1:5 solution during suction filtration) obtained 7.0925 g of solid, the filtrate was spin-dried and weighed to obtain 4.6563 g. That is, the obtained Ugi products totaled 11.7488 g, and the total yield was 73%. The reaction equation is as follows:
[0052]
[0053] Product Confirmation:
[0054] IR (film) 3273, 3085, 2926, 1668, 1...
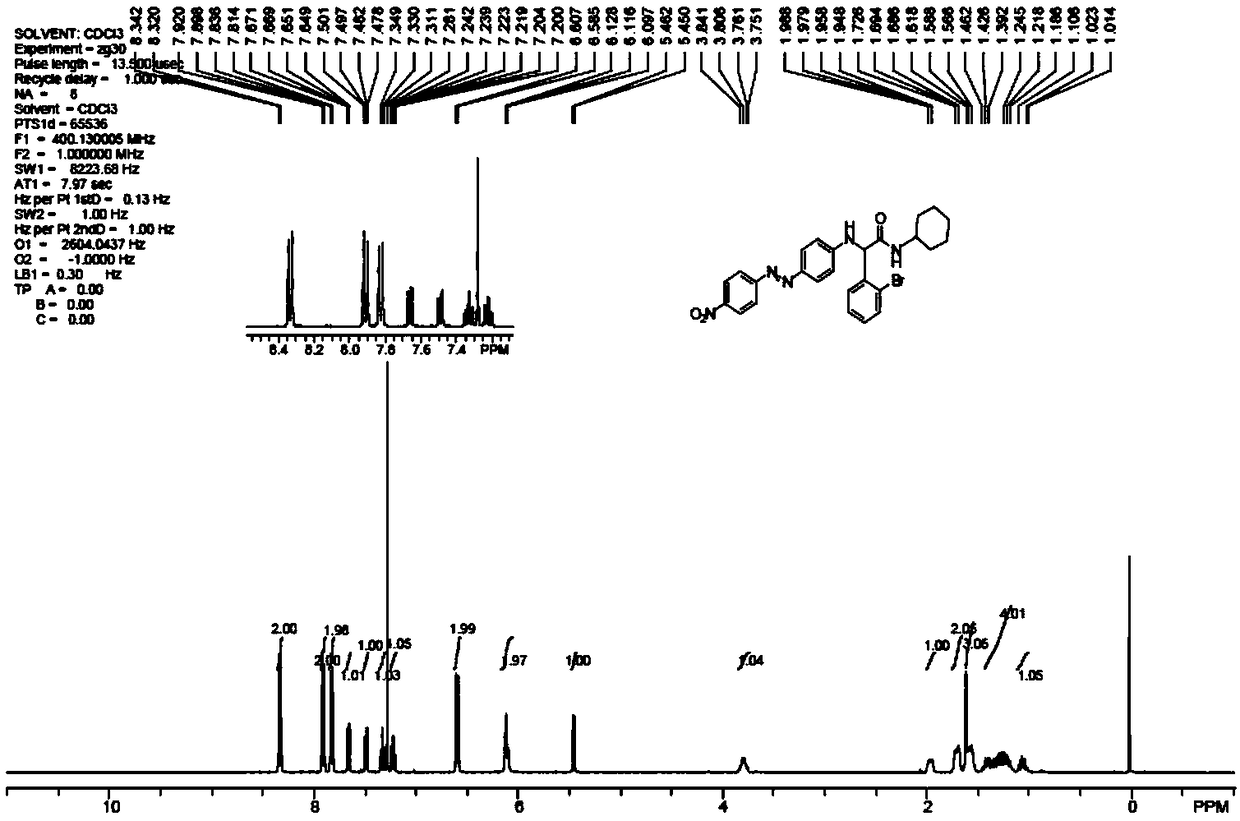
Embodiment 1-2
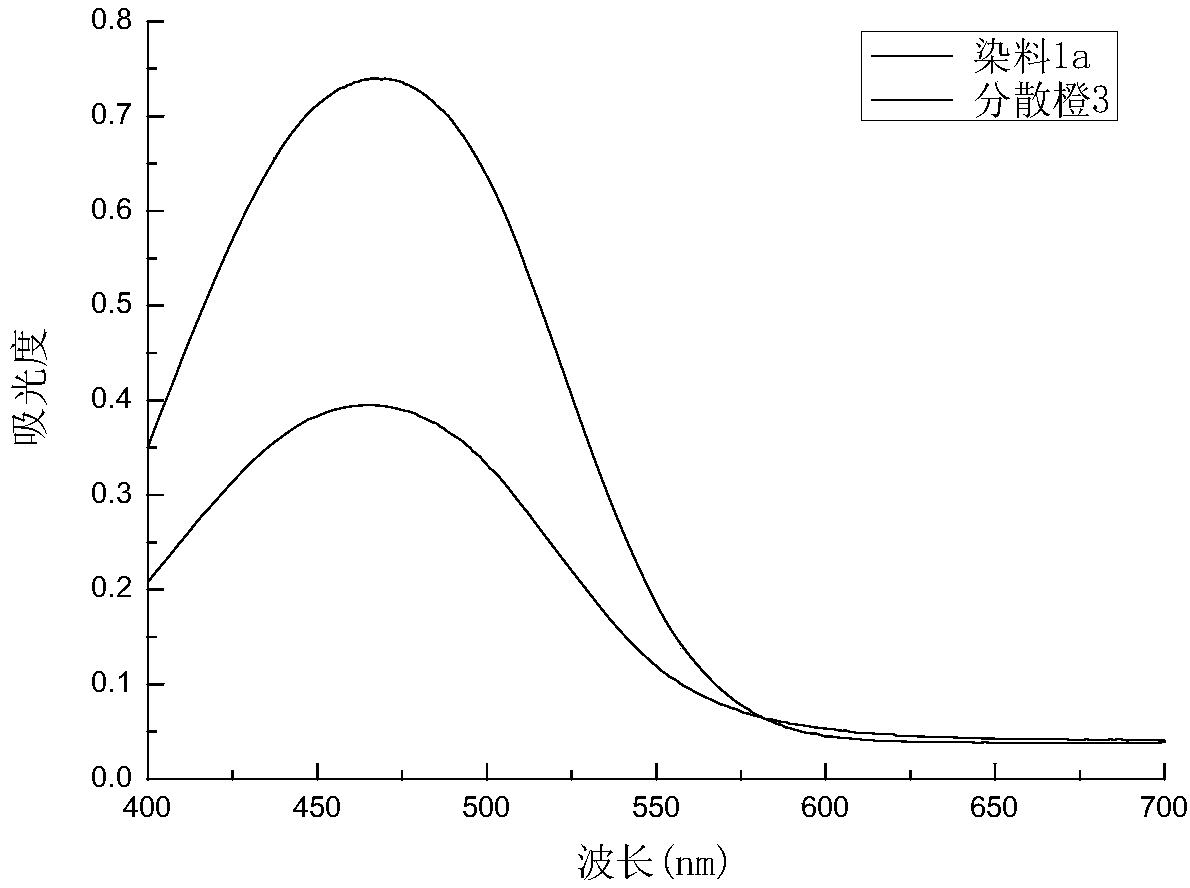
[0074] Spectral absorption performance: Weigh 0.05g of dye 1a, dissolve it in 50mL N,N-dimethylformamide, dilute it by a certain multiple to make the concentration 0.006g / L, and use a UV-2450 ultraviolet-visible spectrophotometer (Japan Shimadzu Corporation) measure the absorption spectrum curve, and compare with the same concentration 0.006g / L Disperse Orange 3 solution absorption spectrum curve, see image 3 .
[0075] From the absorption spectrum curve in the figure, it can be found that compared with Disperse Orange 3, the maximum absorption wavelength of the dye 1a shifted 3nm to the short wavelength direction, from the original 467nm to 464nm. After measurement, the specific performance indicators are shown in the following table 1-1:
[0076] Table 1-1. Performance parameters of dye absorption spectrum
[0077] dye name
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com