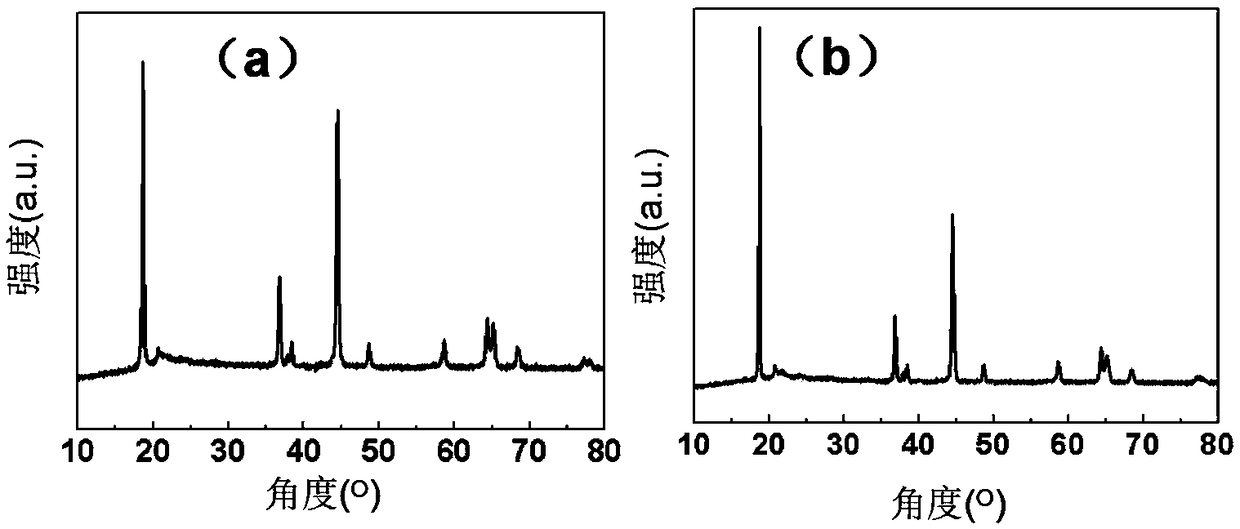
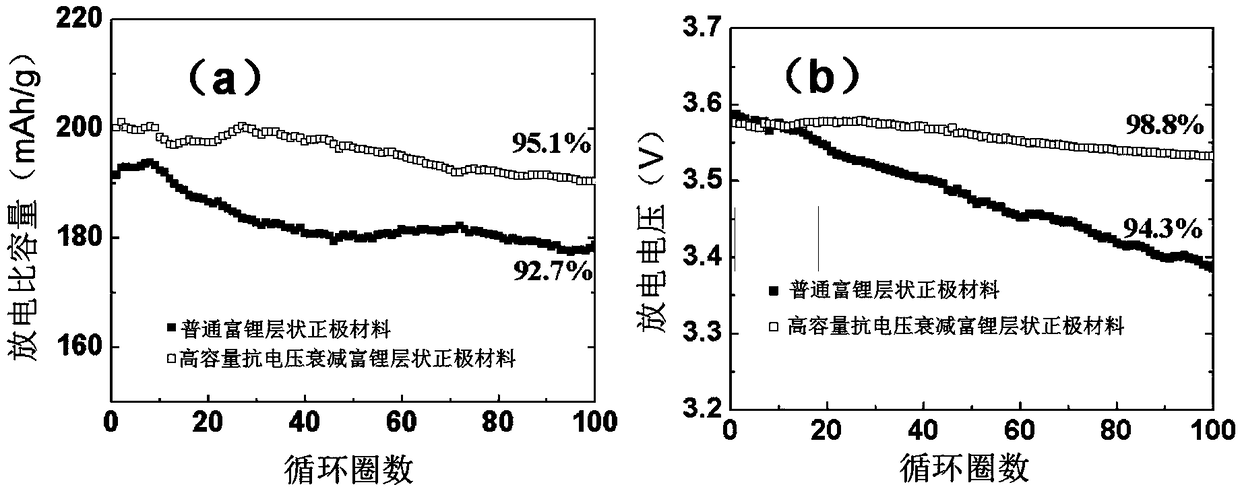
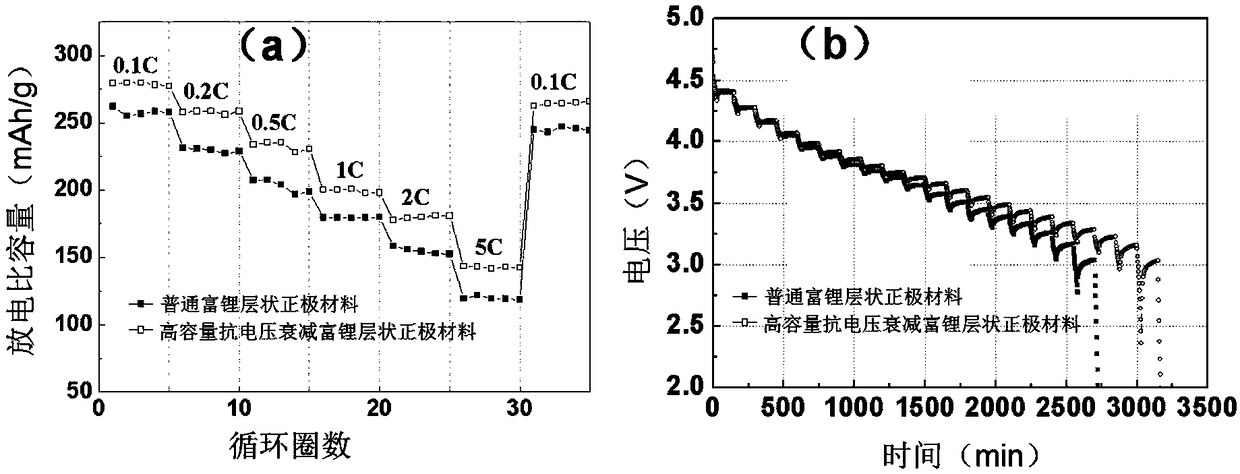
A method for preparing a lithium-rich layered cathode material with high cycle capacity and voltage fading resistance
A technology for positive electrode materials and cycle capacity, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of capacity and voltage decline, anti-voltage decline lithium-rich layered positive electrode materials, etc., to improve voltage decline and increase solid-phase Li+ The effect of transmission ability and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] (1) Preparation of precursor:
[0030] (1-1) Ni salt (NiSO 4 , Ni(NO 3 ) 2 , NiCl 2 , Ni(CH 3 COO) 2 ), Co salt (CoSO 4 , Co(NO 3 ) 2 , CoCl 2 , Co(CH 3 COO) 2 ), Mn salt (MnSO 4 , Mn(NO 3 ) 2 , MnCl 2 , Mn(CH 3 COO) 2 ) dissolved in water, wherein 0≤x≤1, 0≤y≤1, 0≤x+y≤1, so that the total molar concentration of metal ions is ≥1mol / L, and it is ready for use.
[0031] (1-2) Precipitating agent solution: the precipitating agent can be water-soluble oxalate, carbonate, or hydroxide, and the precipitating agent is dissolved in water to make the molar concentration ≥ 1 mol / L for later use.
[0032] (1-3) Configure pH adjuster solution: One or more of ammonia water, sodium hydroxide, sodium carbonate, sodium bicarbonate, ammonium chloride, ammonium carbonate, and ammonium bicarbonate can be used to prepare a pH of 8-12 The pH adjuster solution is ready for use.
[0033] (1-4) Adding the precipitating agent solution into the reactor containing the metal ion ...
Embodiment 1
[0045] (1) According to the molar ratio of 0.163:0.163:0.674 NiSO 4 、CoSO 4 , MnSO 4 Dissolve in water so that the total molar concentration of metal ions is 1mol / L, and set aside. Prepare the precipitant solution: the precipitant is sodium carbonate, dissolve the precipitant in water so that the molar concentration is 1mol / L, and set aside. Prepare the pH regulator solution: use ammonia water and sodium carbonate to prepare a pH regulator solution with a pH of 11, and set aside. Add the precipitant solution into the reaction kettle containing the mixed solution of metal ions. During the reaction, add a pH regulator solution to adjust the pH to 10.5, and stir at the same time. After the reaction is complete, the precipitate produced is centrifuged, washed with deionized water and ethanol, and dried in a blast oven to obtain Precursor, the molecular formula of the precursor is: Ni 0.163 co 0.163 mn 0.674 CO 3 ;
[0046] (2) According to the precursor Ni 0.163 co 0.16...
Embodiment 2
[0052] (1) According to the molar ratio of 0.25:0.75, NiSO 4 , MnSO 4 Dissolve in water (at this time, the value of y in the Co salt is 0, the same below), so that the total molar concentration of metal ions is 2mol / L, and set aside. Prepare the precipitant solution: precipitant oxalic acid, dissolve the precipitant in water to make the molar concentration 2mol / L, set aside. Add the precipitant solution into the reaction kettle containing the mixed solution of metal ions, and stir at the same time. After the reaction is complete, centrifuge the generated precipitate, wash the precipitate with deionized water and ethanol, and place the precipitate in a blast oven Dried to obtain the precursor, the molecular formula of the precursor is: Ni 0.25 mn 0.75 C 2 o 4 2H 2 O;
[0053] (2) According to the precursor Ni 0.25 mn 0.75 C 2 o 4 2H 2 O: The molar ratio of the metal element Ni+Mn that needs to be added during calcination (the source of Ni is nickel acetate, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com