Porous polymeric sorbent for carbon dioxide
A porous polymer and carbon dioxide technology, applied in alkali metal compounds, alkali metal oxides/hydroxides, inorganic chemistry, etc., can solve problems such as expensive energy and chemical solvent heating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1B
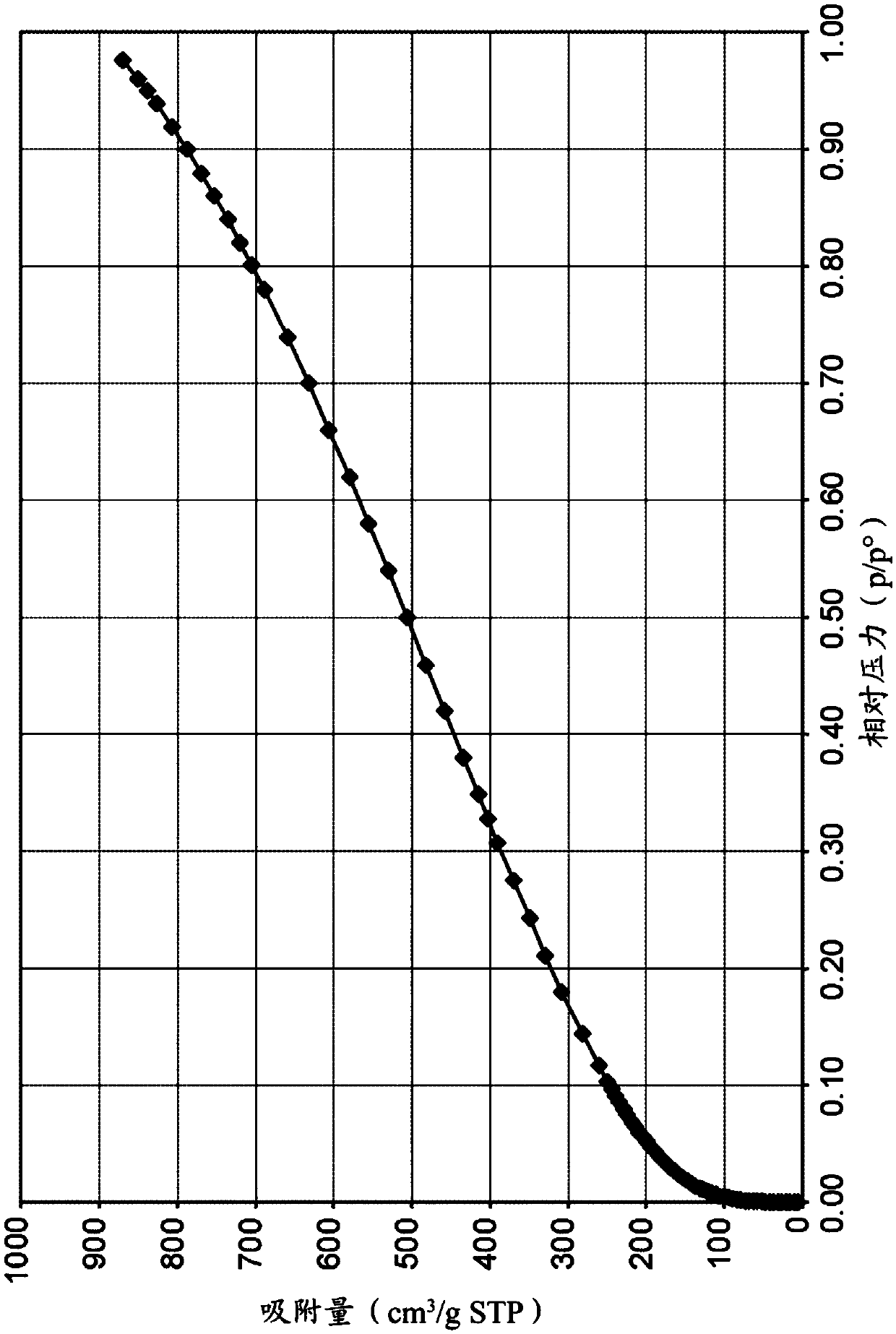
[0095] Embodiment 1B is a composition comprising: a) a porous polymeric adsorbent having a mass equal to at least 400 m 2 / g of BET specific surface area, and b) carbon dioxide adsorbed on a porous polymer adsorbent wherein at least 1.0 mmol of carbon dioxide is adsorbed at 5 bar and 25°C. The porous polymeric adsorbent contains: a) 75% to 100% by weight of the first monomer unit of formula (I),
[0096]
[0097] and b) 0% to 25% by weight of a second monomer unit of formula (II),
[0098]
[0099] In formula (II), R 1 is hydrogen or alkyl. Each weight % value is based on the total weight of the porous polymeric adsorbent. Each asterisk (*) (here and throughout) indicates the attachment position of that monomeric unit to another monomeric unit or end group.
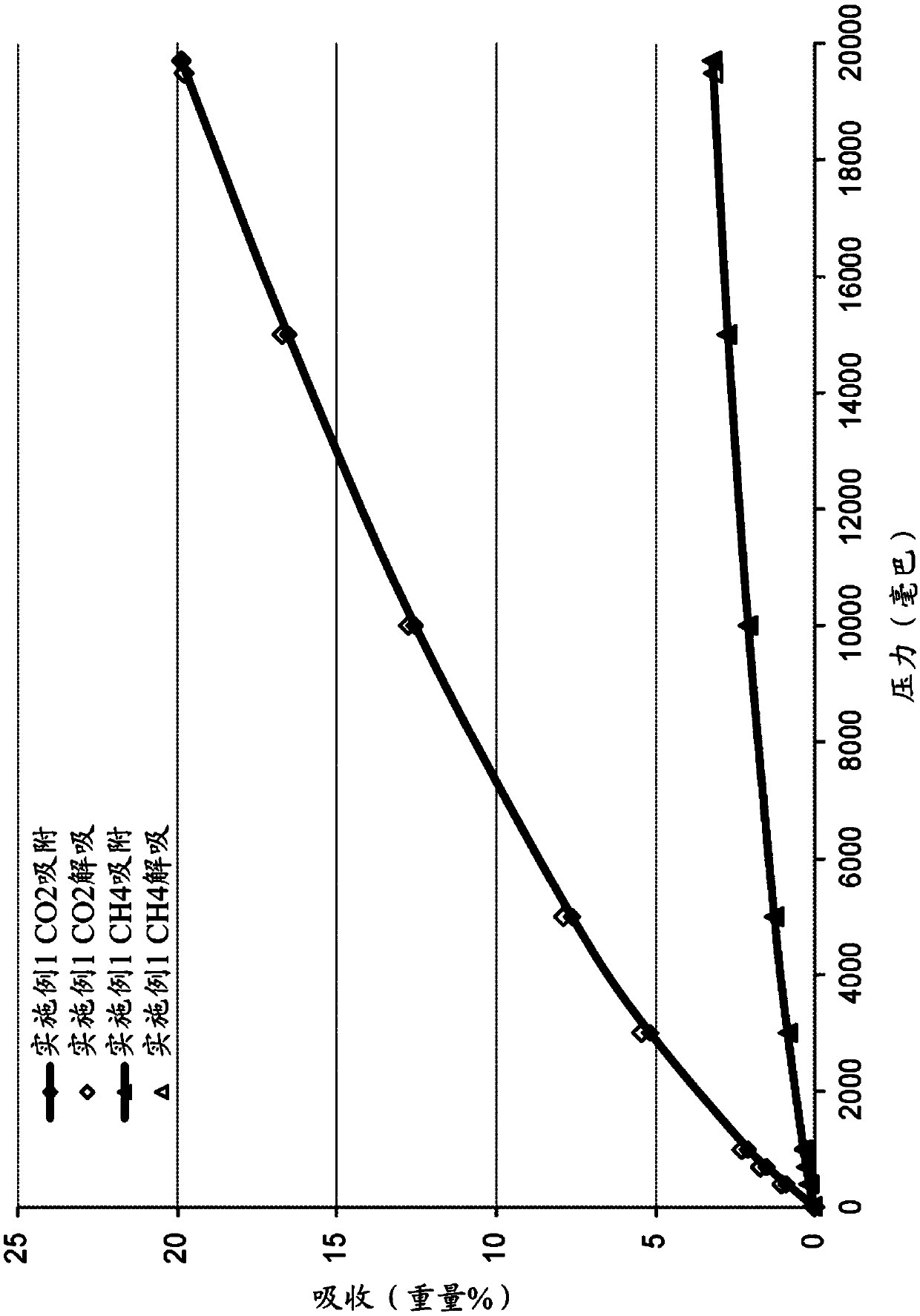
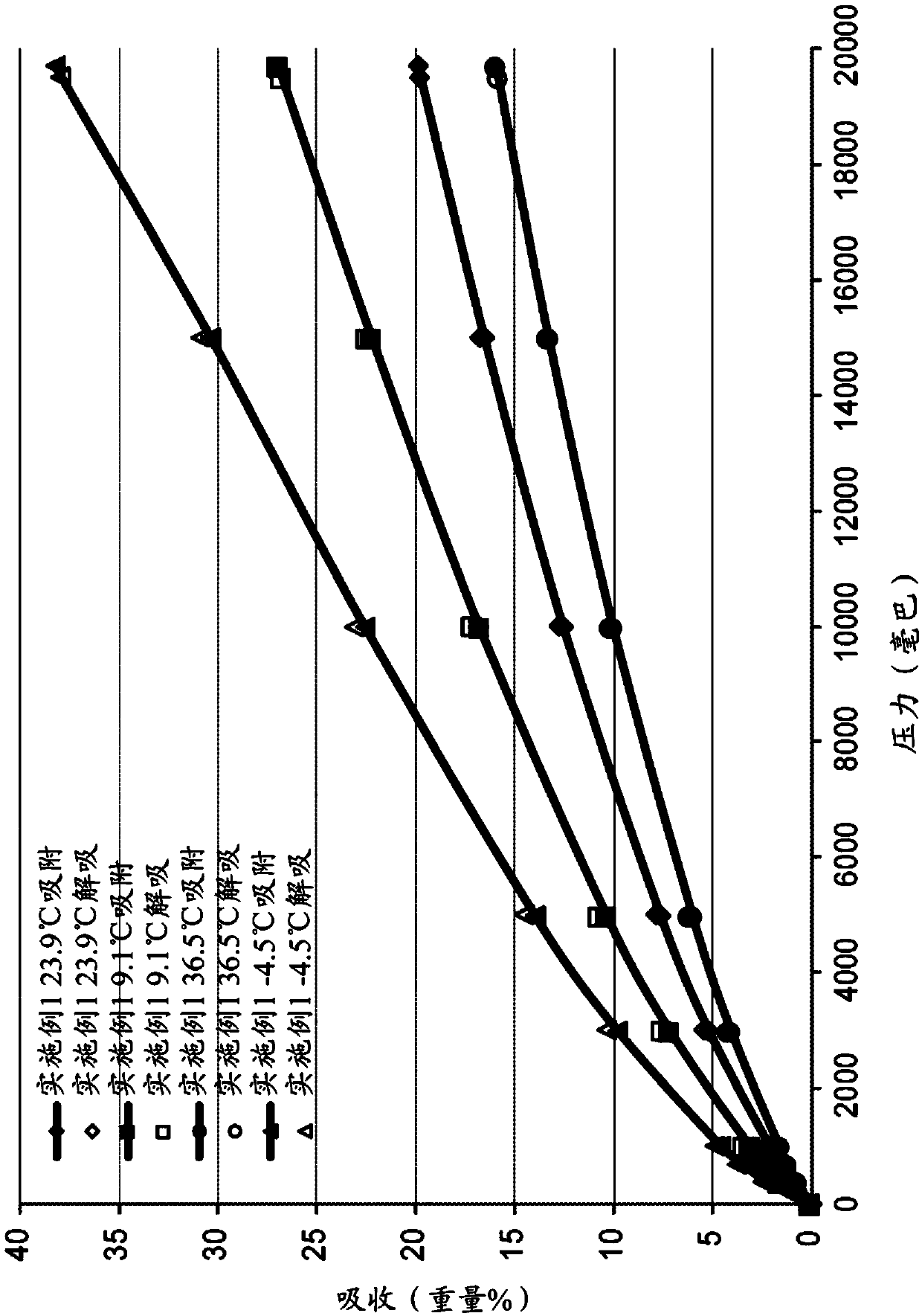
[0100] Embodiment 2B is the composition of embodiment 1 B, wherein at least 1.5 millimoles of carbon dioxide is adsorbed at 10 bar and 25°C.
[0101] Embodiment 3B is the composition of embodiment 1B or 2B, whe...
Embodiment 1
[0118] In a 5 L round bottom flask, dissolve 125.0 g (768 mmol) divinylbenzene (DVB) (80%, technical grade) and 2.50 g (10.3 mmol) benzoyl peroxide (BPO) in 2.65 L acetic acid in ethyl ester (EtOAc). The polymerizable composition had a percent solids of 5.0% by weight in EtOAc and contained a monomer mixture (80.0% by weight DVB and 20.0% by weight styrenic monomer) and 2% by weight BPO (based on the total weight of monomers). Nitrogen was bubbled through the polymerizable composition for 30 minutes. The flask was then capped and placed in a 95°C sand bath. The polymerizable composition was heated at this elevated temperature for 17 hours. The white precipitate formed was isolated by vacuum filtration and washed with EtOAc. The solid was dispensed and placed into two 32 oz jars. Each jar was then filled with 700 mL of EtOAc. The solid was allowed to stand in EtOAc for 1 h at room temperature. The solid was isolated again by vacuum filtration and washed with EtOAc. The s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com