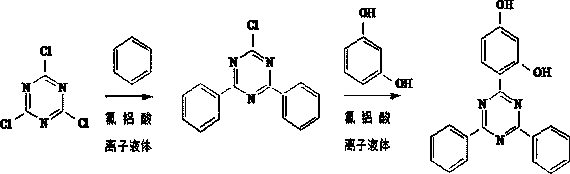
Method for preparing 2-(2,4-dihydroxyphenyl)-4,6-diphenyl-1,3,5-triazine
A technology of dihydroxyphenyl and diphenyl, which is applied in the field of preparation of 2--4,6-diphenyl-1,3,5-triazine, can solve the problem of lack of economic attractiveness, difficulty in recycling, Long synthetic route and other problems, to avoid the pressure of three wastes treatment, overcome the non-reusable, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In the 250ml reaction flask, add 40g 1-butyl-3-methylimidazolium chloroaluminate ionic liquid ( [Bmim]Cl-2AlCl 3 ) and 18.45g (0.1mol) cyanuric chloride, add 15.6g (0.2mol) benzene dropwise under stirring condition at 15~20°C, finish adding in 4h, and keep warm at 15~20°C for 24h after dropping, with 210nm wavelength Under the condition of liquid chromatographic analysis, the content of cyanuric chloride is 1.5%.
[0024] The temperature of the above reaction solution was raised to 80°C, and 11 g (0.1 mol) of resorcinol was added in batches, and the addition was completed within 2 hours, during which a yellow solid was precipitated. After the addition, the reaction was kept for 4 hours.
[0025] Add 100ml of o-dichlorobenzene into the reaction bottle, stir well at 80°C, keep warm and let it stand for stratification. The chloroaluminate ionic liquid in the lower layer is recovered for the next reaction and reused. After the upper layer is cooled, a solid is precipitated...
Embodiment 2
[0027] In the 250ml reaction bottle, add 80g by the 1-ethyl-3-methylimidazolium chloroaluminate ionic liquid ( [Emim]Cl-1.8AlCl 3 ) and 18.45g (0.1mol) cyanuric chloride, add 16.38g (0.21mol) benzene dropwise under stirring condition at 20~25°C, add it in 4h, and keep it at 20~25°C for 12h after the dropwise addition, 210nm wavelength Under the condition of liquid chromatography analysis, the content of cyanuric chloride is 1.8%.
[0028] The temperature of the above reaction solution was raised to 75°C, and 9.9 g (0.09 mol) of resorcinol was added in batches, and the addition was completed within 2 hours, during which a yellow solid was precipitated. After the addition, the reaction was kept for 6 hours.
[0029] Add 100ml of chlorobenzene into the reaction flask, stir well at 75°C, keep warm and let stand for stratification. The chloroaluminate ionic liquid in the lower layer is recovered for the next reaction and reused. After the upper layer is cooled, the solid is precip...
Embodiment 3
[0031] In the 250ml reaction bottle, add 100g by aluminum trichloride and 1-butyl-4-picoline chloride 1-butyl-4-picoline molar ratio 2.2:1 mix the 1-butyl-4-picoline chloroaluminate ionic liquid that obtains ( [Bmp]Cl-2.2AlCl 3 ) and 18.45g (0.1mol) cyanuric chloride, add 14.04g (0.18mol) benzene dropwise under stirring condition at 25~30°C, finish adding in 2h, and keep warm for 8h at 25~30°C after dropping, at 210nm wavelength Under the condition of liquid chromatographic analysis, the content of cyanuric chloride was 1.6%.
[0032] The temperature of the above reaction solution was raised to 85°C, and 12.1 g (0.11 mol) of resorcinol was added in batches, and the addition was completed within 4 hours, during which a yellow solid was precipitated, and after the addition was completed, the reaction was kept for 8 hours.
[0033] Add 80ml of o-dichlorobenzene into the reaction bottle, stir well at 85°C, keep it warm and let it stand for stratification. The chloroaluminate ioni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com