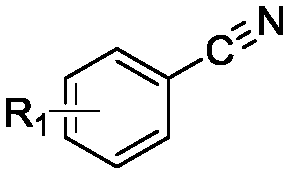
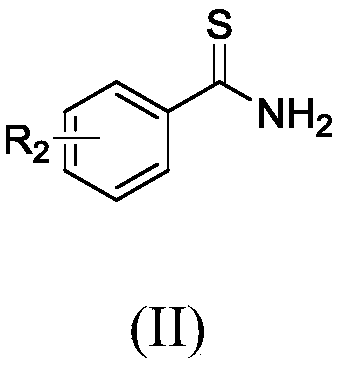
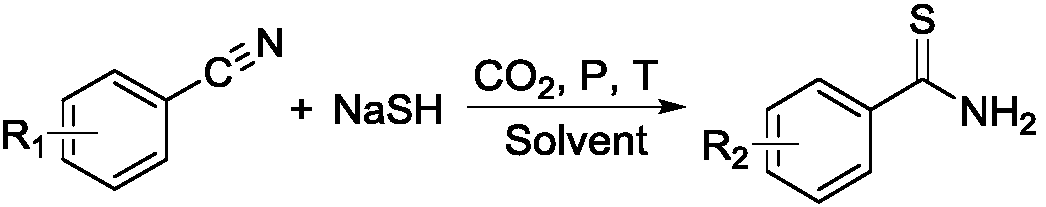Method for synthesizing thiobenzamide derivative through CO2 regulation and control of substituted benzonitrile
A technology for synthesizing thiobenzamide and thiobenzamide, which is applied in the direction of organic chemistry and the like, can solve the problems of high reaction cost, many by-products in the reaction process, low conversion rate and the like, and achieves a simple reaction system and wide adaptability. , the effect of easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0037] Example 1-15: Synthesis of o-fluorobenzothiobenzamide with o-fluorobenzonitrile
[0038] Put a magnet into a 10mL stainless steel autoclave, and add 1mmol of o-fluorobenzonitrile, an appropriate amount of inorganic sulfide and 2ml of solvent in sequence, and tighten the autoclave. Fill the reactor with 0.1MPa of CO 2 , Stir the reaction for 2h, after the reaction is completed, slowly exhaust the gas in the reaction kettle, unscrew the reaction kettle, and transfer the solution in the reaction kettle to a 50mL Erlenmeyer flask. After adding saturated brine, it was extracted with ethyl acetate, and the organic phases were combined and dried over anhydrous magnesium sulfate for 30 minutes. Anhydrous magnesium sulfate was removed by filtration, and the crude product was obtained by distillation under reduced pressure. Use a mixed solvent of petroleum ether and ethyl acetate as eluent, separate and concentrate through column chromatography (200-300 mesh silica gel) to obta...
Embodiment 16~24
[0044] Examples 16-24: Synthesis of thiobenzamide derivatives.
[0045] The following thiobenzamide derivatives were synthesized with reference to the optimal conditions of o-fluorothiobenzamide.
[0046] Among them: the temperature is 25°C, the pressure is 0.1MPa, o-fluorobenzonitrile: inorganic sulfide = 1:2, the molar ratio of o-fluorobenzonitrile and carbon dioxide is 1:0.3; the solvent is DMF, the inorganic sulfide is NaHS, and the reaction time 24h.
[0047] The relevant data are listed in Table 2.
[0048]
[0049] Table 2: Synthesis of thiobenzamide derivatives
[0050]
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com