SBS containing 1,2 structure uniformly distributed butadiene block and hydride thereof and preparation and application method thereof
A technology of uniform distribution and butadiene, applied in SBS containing 1,2 structure uniform distribution of butadiene block and its hydride, preparation and application, can solve the problem of uneven distribution of butadiene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
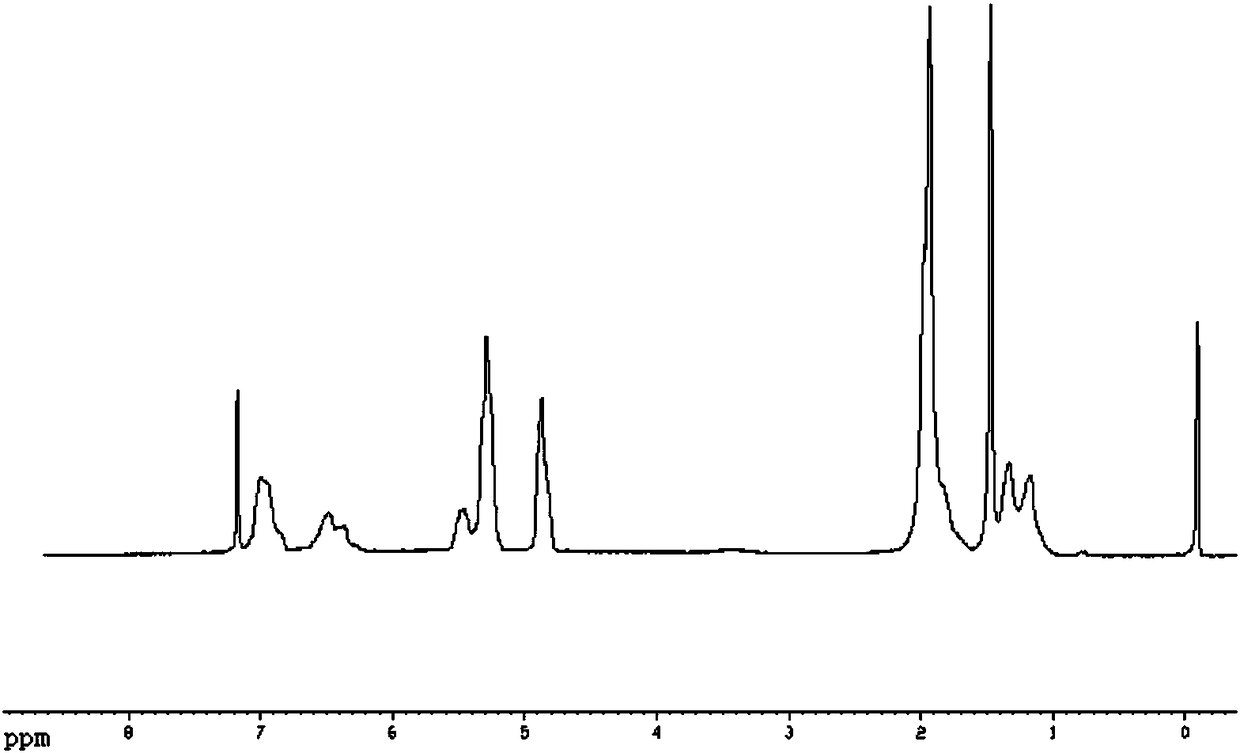
Examples
Embodiment 1
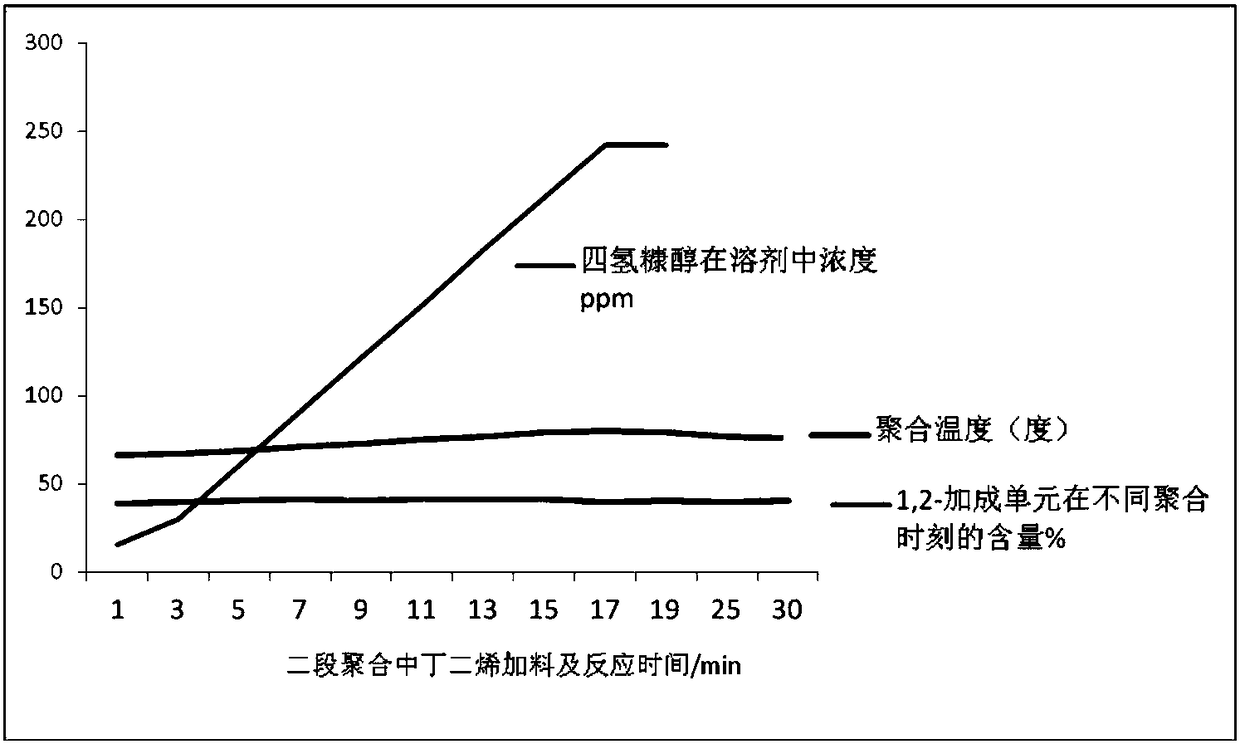
[0056] Add 3500mL of cyclohexane solution with a mass fraction of 10% n-hexane in a 5-liter steel-made polymerization kettle under nitrogen protection, then simultaneously inject THF 1.5g and styrene 50mL into the polymerization kettle with a syringe. After the temperature of the solvent rises to 55-65°C, inject 6.5mL of 0.5mol / L n-butyllithium into the polymerization kettle with a syringe to initiate the first stage of polymerization. The polymerization pressure is 0.2-0.3MPa. After 23-25min of polymerization, the When the initial temperature of the second stage is controlled at 66 ° C, 340 mL of butadiene solution mixed with 0.95 mL tetrahydrofurfuryl alcohol ethyl ether in the butadiene metering tank is uniformly pressed into the polymerization kettle within 16 minutes with nitrogen gas, and if necessary, the Add cooling water to control the temperature rise rate, that is, the temperature rise rate is controlled at (0.5-1) °C / min, and the second-stage polymerization temperat...
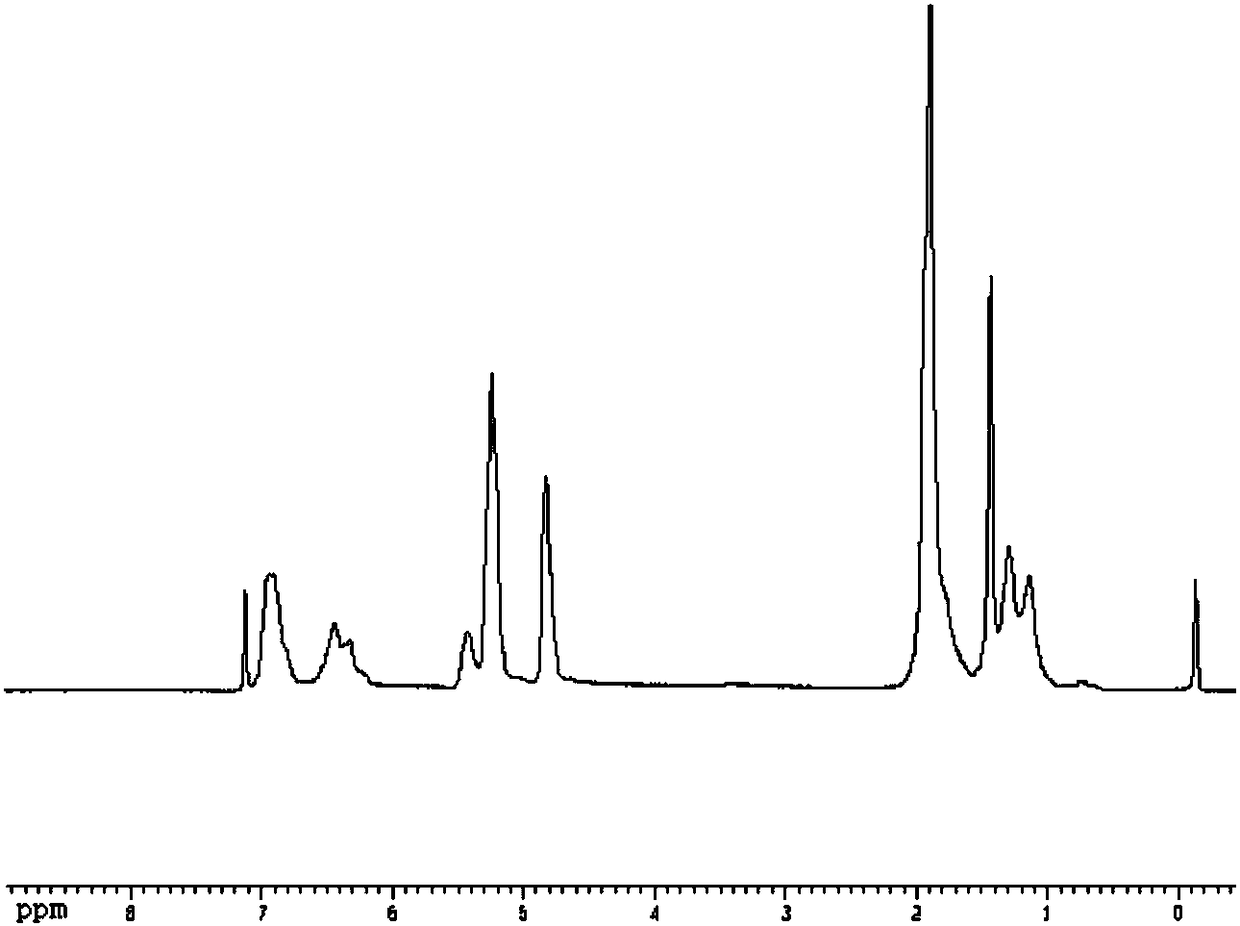
Embodiment 2
[0061] With the process conditions in Example 1 unchanged, only the styrene of the first section and the third section polymerization is all set to 55mL, the n-butyllithium 4mL that the first section initiates is used, and activator selects DTHFP for use, and its consumption 0.28mL, the second stage uses 325mL of butadiene, DTHFP is 1.0mL when it is miscible in butadiene, the initial temperature of the second stage is controlled at 70°C, and the butadiene solution of ditetrahydrofurfuryl propane is heated within 18 minutes with nitrogen gas Press evenly into the polymerization kettle.
[0062] Results: The content of 1,2-addition units in polybutadiene units in different polymerization time periods in the butadiene chain growth stage during the polymerization process, and the corresponding polymerization temperature are shown in Table 1.
[0063] Table 1 Corresponding relationship between 1,2-addition unit content and temperature in different polymerization stages of butadiene...
Embodiment 3
[0069] With the process conditions in Example 1 constant, the styrene of the first section and the third section polymerization is all set as 55mL, the n-butyllithium 5mL that the first section initiates is used, and activator selects tetrahydrofurfurylamine for use, and its consumption 0.35mL, 325mL of butadiene is used in the second stage, 1.0mL of tetrahydrofurfurylamine is miscible in butadiene, the initial temperature of the second stage is controlled at 68°C, and the butadiene solution of tetrahydrofurfurylamine is filled with nitrogen in the Press evenly into the polymerization kettle within 18 minutes.
[0070] Results: Table 2 shows the content of 1,2-addition units in polybutadiene units at different polymerization time periods in the butadiene chain growth stage and the corresponding polymerization temperature in the polymerization process.
[0071] Table 2 Corresponding relationship between 1,2-addition unit content and temperature in different polymerization stage...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com