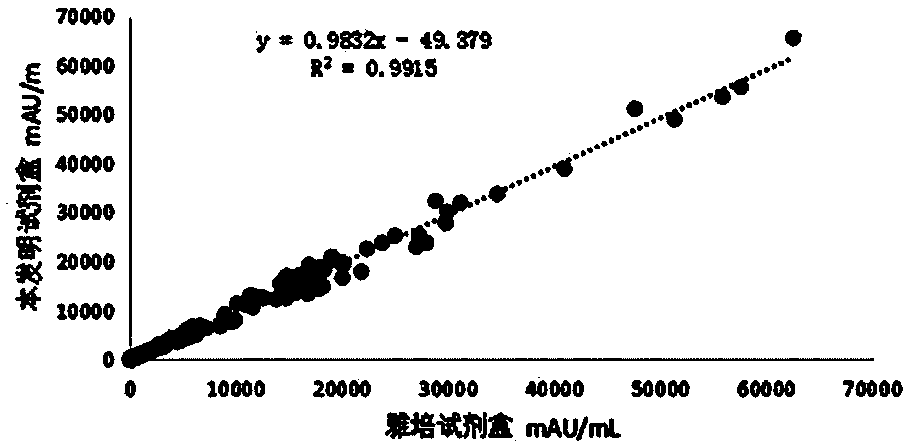
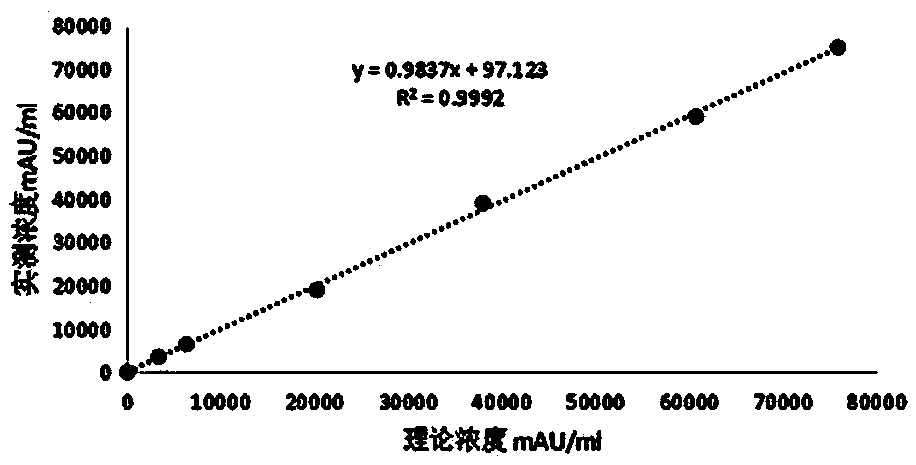
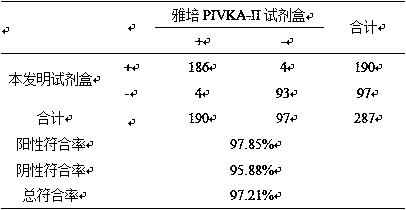
Kit for clinical detection of abnormal prothrombin
A prothrombin, abnormal detection technology, applied in the field of medical testing, can solve the problems of inconvenient use, high price, short validity period, etc., and achieve the effects of avoiding detection errors, wide detection range and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of kits for clinical detection of abnormal prothrombin
[0029] 1. Prepare sealing solution
[0030] The sealing solution is prepared from 0.01 M PBS buffer solution with pH 7~8, which contains 1~3‰ (w / v) preservative (Proclin300, MIT, Bro, IPBC or NaN 3 One or a mixture of two or more of them), 1~3% (w / v) protein stabilizer (one or two or more of casein, fish gelatin or bovine serum albumin can be used) and 0.1 ~1% (w / v) glycerin.
[0031] 2. Prepare enzyme conjugate dilution
[0032] The enzyme conjugate is prepared by 0.05 M Tris-NaCl buffer solution with pH 7~8, which contains 1~3‰ (w / v) preservative (Proclin 300, MIT, Bro, IPBC or NaN can be used 3 1~3% (w / v) protein stabilizer (one or more mixtures of casein, fish gelatin or bovine serum albumin can be used) and 0.1 ~0.5‰ of carmine pigment.
[0033] 3. Prepare sample diluent
[0034] The sample diluent is prepared by 0.01 M PBS buffer solution with pH 7~8, which contains 1~3‰ (w / v) pre...
Embodiment 2
[0049] Embodiment 2 Sensitivity assessment of the kit of the present invention
[0050] LoB, LoD, and LoQ were established in accordance with the performance confirmation (establishment) method of the CLSI standard "EP17-A: Evaluation of Clinical Laboratory Detection Limits". Items that cannot be strictly traced to SI units do not establish LoQ, but establish the functional sensitivity (FS) of the kit.
[0051] Limit of Blank (LoB): Prepare 5 clinical samples with a value close to 0, repeat 3 times for each sample, do a total of 4 days, get 60 data, and calculate LoB according to the calculation method of EP17-A.
[0052] Limit of detection (LoD): Prepare 5 serial clinical samples with a concentration range of 1 to 4 times the LOB, repeat 3 times for each sample, and do a total of 4 days to obtain 60 data, and calculate the LoD according to the calculation method of EP17-A.
[0053] Functional sensitivity (FS): Using the data in the LoD experiment, 5 concentration samples wer...
Embodiment 3
[0057] Embodiment 3 The precision assessment of the kit of the present invention
[0058] Three batches of reagents were taken for precision experiments, and high / low value quality control products were used for assessment respectively, and the variation of the measured concentration was calculated 20 times for each measurement. The measurement results are shown in Table 2 below, and the results show that the coefficients of variation are all less than 6%.
[0059] Table 2 Kit precision
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com