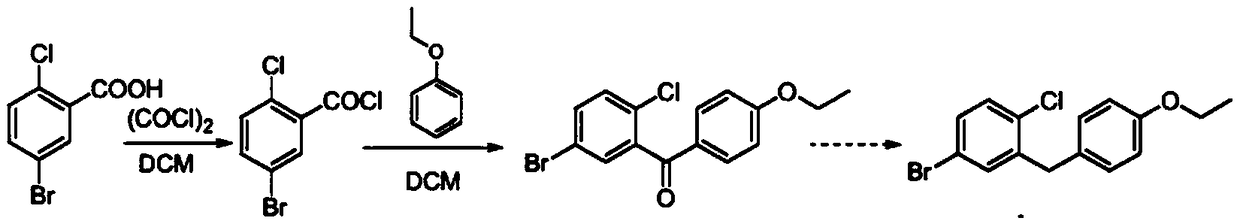
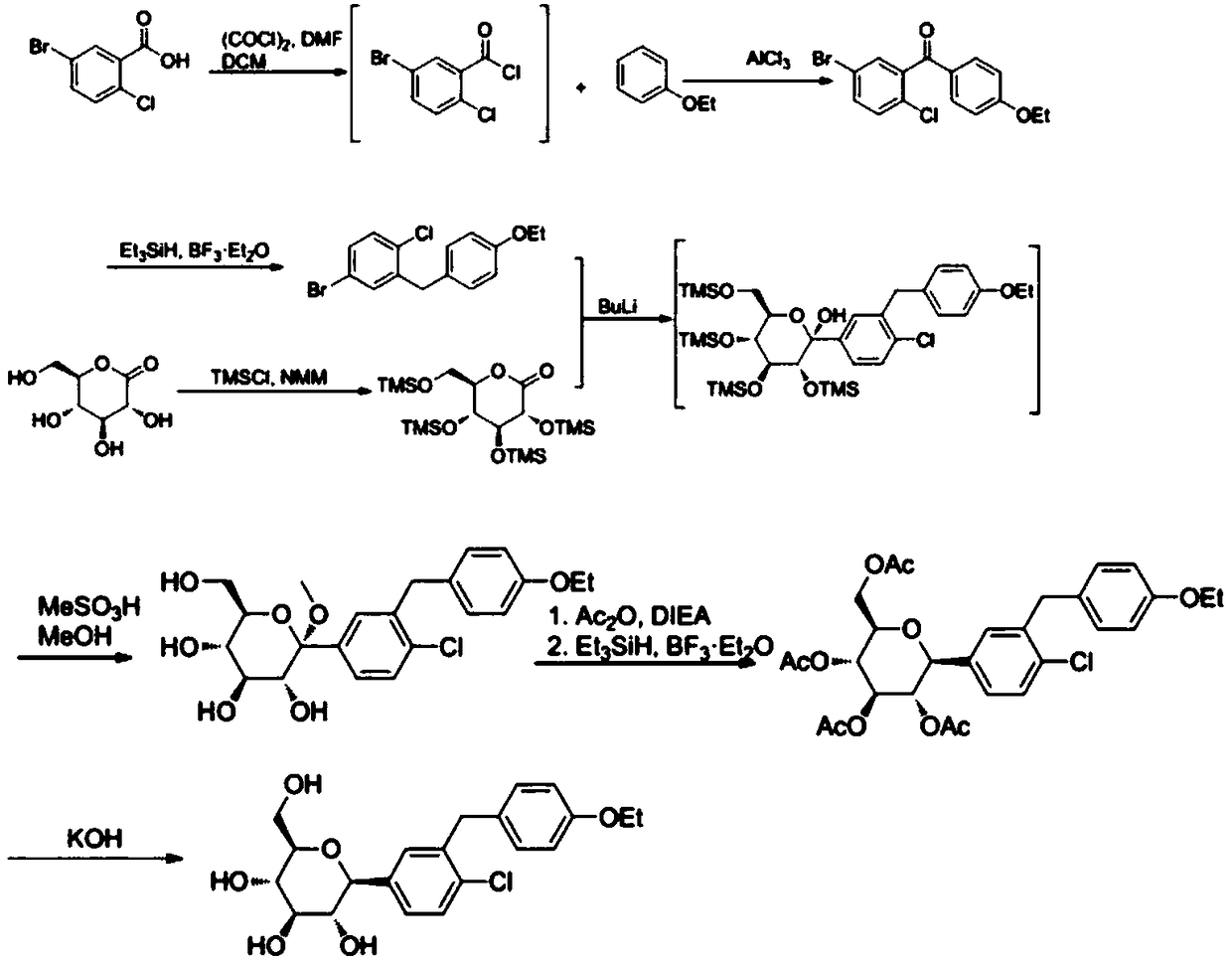
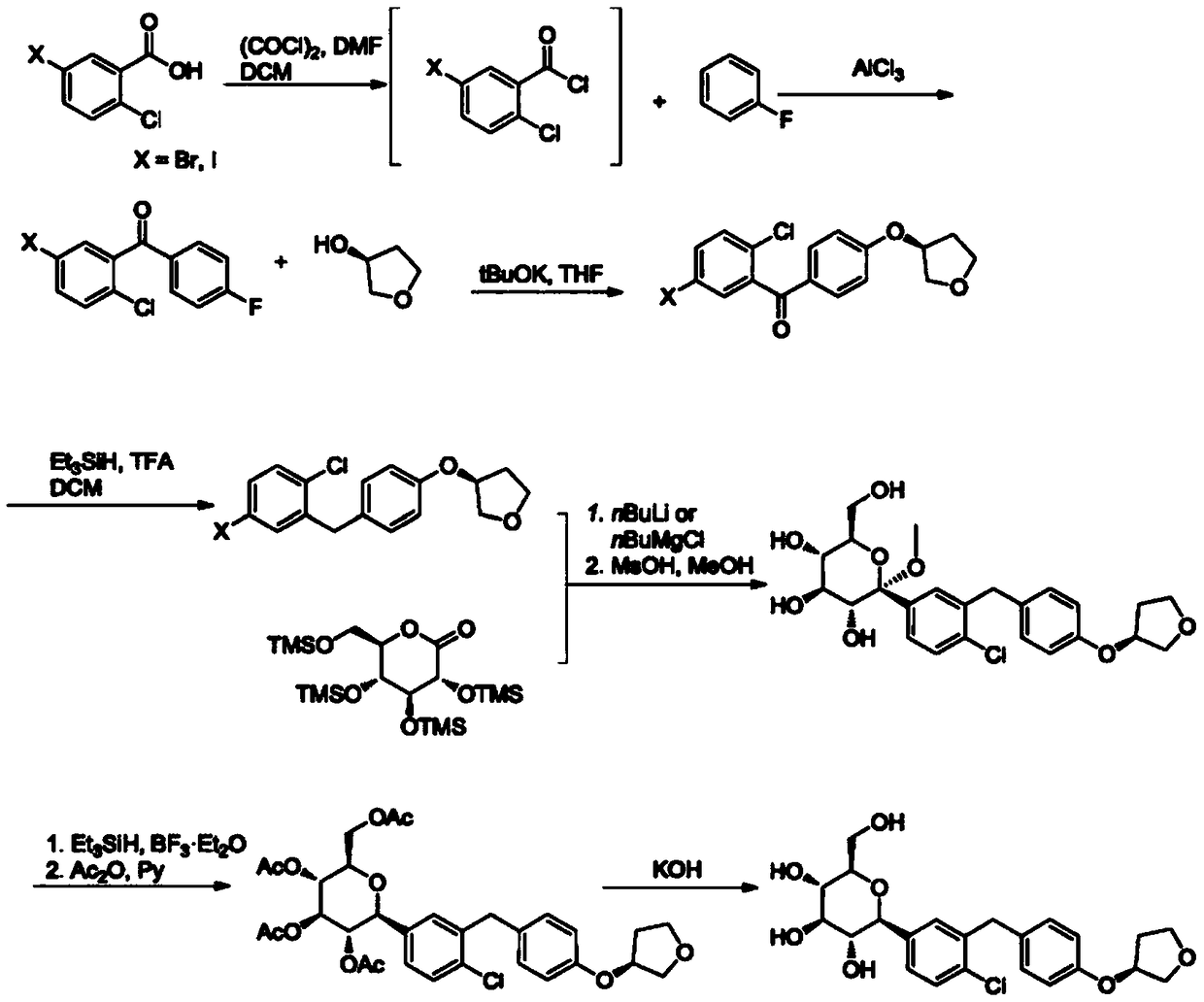
Synthetic method of 1-chloro-2-(4-ethoxybenzyl)-4-iodobenzene
A technology of ethoxybenzyl and iodobenzene, which is applied in the field of drug synthesis, can solve the problems of too long reaction steps, prone to danger, unfavorable large-scale production, etc., and achieve the effect of short synthetic route and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Mix 5.0g of 4-bromophenetole and 100mL of tetrahydrofuran, stir until clear, and set aside.
[0030] (2) Add 0.64g of magnesium chips and 100mL of tetrahydrofuran in sequence, stir and mix thoroughly, pour 20mL of 4-bromophenetole in tetrahydrofuran into it, heat up to 45°C, stop stirring after reaching the specified temperature, and add 0.63g of elemental iodine Particles, the reaction is triggered, and bubbles overflow from the bottom of the system. When a large number of bubbles overflow, start stirring slowly until the system changes from dark red to colorless, clear and transparent, and the temperature of the system increases significantly.
[0031] (3) After adding 0.63g of elemental iodine particles, the normal rotation speed was restored, and the remaining tetrahydrofuran solution of 4-bromophenetole was added dropwise. The temperature of the system was maintained at a slight reflux state, and the dropwise addition was completed in 1-1.5 hours. After droppi...
Embodiment 2
[0035] (1) Mix 5.0g of 4-bromophenetole and 100mL of diethyl ether, and stir until clear. spare.
[0036] (2) Add 0.67g of magnesium chips and 100mL of diethyl ether in sequence, stir and mix thoroughly, pour 20mL of 4-bromophenetole ether solution into it, heat up to 50°C, stop stirring after reaching the specified temperature, and add 0.63g of elemental The iodine particles trigger the reaction, and bubbles overflow from the bottom of the system. When a large number of bubbles overflow, start stirring slowly until the system changes from dark red to colorless, clear and transparent, and the temperature of the system increases significantly.
[0037] (3) After adding 0.63g of elemental iodine particles, the normal rotation speed was restored, and the remaining ether solution of 4-bromophenetole was added dropwise. The temperature of the system was maintained at a slight reflux state, and the dropwise addition was completed in 1-1.5 hours. After dropping, keep microreflux re...
Embodiment 3
[0041] (1) Mix 5.0g of 4-bromophenetole with 100mL of a 1:1 mixed solution of diethyl ether and tetrahydrofuran, and stir until clear. spare.
[0042] (2) Add 0.69g of magnesium chips and 100mL of a mixed solution of diethyl ether and tetrahydrofuran (1:1) in sequence, start stirring and mix thoroughly, pour 20mL of 4-bromophenetole solution into it, heat up to 48°C, and reach the specified temperature , stop stirring, add 0.63g elemental iodine particles to initiate the reaction, and bubbles overflow from the bottom of the system. When a large number of bubbles overflow, start stirring slowly until the system changes from dark red to colorless, clear and transparent, and the temperature of the system increases significantly.
[0043](3) After adding 0.63g of elemental iodine particles, the normal rotation speed was restored, and the remaining 4-bromophenetole solution was added dropwise. The temperature of the system was maintained at a slight reflux state, and the dropwise ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com