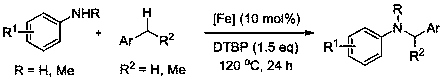
Method of preparing benzylamine compound
The technology of a compound and benzylamine is applied in the field of preparation of organic compounds, and achieves the effect of wide application range, being conducive to large-scale industrial synthesis application and improving applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment one: containing 1,3-di-tert-butylimidazolium cation (molecular formula is [( t BuNCHCHN t Bu)CH][FeBr 4 ]) Synthesis of ionic iron complexes
[0028] Add 1,3-di-tert-butylimidazolium bromide (0.26 g, 1.0 mmol) to a tetrahydrofuran solution of iron tribromide (0.27 g, 0.9 mmol), react at 60°C for 24 hours, and remove the solvent in vacuo , washed with hexane, drained, extracted with tetrahydrofuran, centrifuged to transfer the supernatant, added hexane to the supernatant for recrystallization, and reddish-brown crystals were precipitated at room temperature with a yield of 89%.
[0029] The product is subjected to elemental analysis, and the results are as follows:
[0030] Elemental analysis
[0031]
C:(%)
H:(%)
N:(%)
theoretical value
23.73
3.80
5.03
actual value
23.88
3.89
5.14
[0032] This complex [( t BuNCHCHN t Bu)CH][FeBr 4 ] exists in the form of ion pairs, where [FeBr 4 ] - Characterize...
Embodiment 2
[0036] Embodiment two: [( t BuNCHCHN t Bu)CH][FeBr 4 ] Catalyzed reaction of p-cyanoaniline with toluene
[0037] Add p-cyanoaniline (59 mg, 0.5 mmol), catalyst (28 mg, 0.05 mmol), di-tert-butyl peroxide (138 μl, 0.75 mmol) and toluene (7 ml) in turn in the reaction flask React at 120°C for 24 hours, cool to room temperature after the reaction, and purify the product by column chromatography (using a mixed solvent of ethyl acetate / petroleum ether volume ratio of 1:50 as the developing solvent), and the yield is 88%.
[0038] When the catalyst is ferric bromide (10mol%), the yield is only 8%; when the oxidant is tert-butyl hydroperoxide (1.5 times), the yield is only 22%.
[0039] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 , TMS): 7.38-7.28 (m, 7H), 6.58-6.55 (m, 2H), 4.73(s, 1H), 4.35 (s, 2H) ppm.
Embodiment 3
[0040] Embodiment three: [( t BuNCHCHN t Bu)CH][FeBr 4 ] Catalyzed reaction of p-cyanoaniline with p-tert-butyltoluene
[0041] Add p-cyanoaniline (59 mg, 0.5 mmol), catalyst (14 mg, 0.025 mmol), di-tert-butyl peroxide (138 μl, 0.75 mmol) and p-tert-butyltoluene in the reaction flask in sequence (7 milliliters) was reacted at 80°C for 60 hours, cooled to room temperature after the reaction, and the product was purified by column chromatography (using ethyl acetate / petroleum ether volume ratio of 1:50 mixed solvent as developing solvent), the yield 86%.
[0042] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl3, TMS): 7.45 (m, 4H), 7.32 (d, J = 7.9 Hz, 2H),6.69-6.60 (m, 2H), 4.65 (s, 1H), 4.39 (s, 2H), 1.38 (s, 9H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com