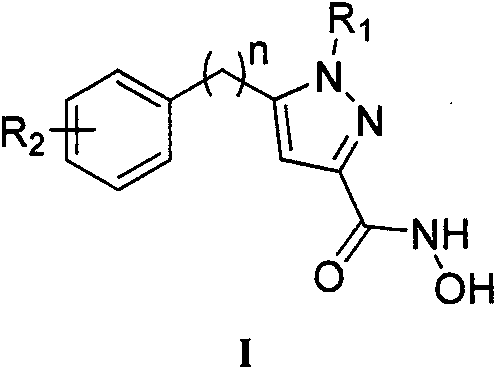
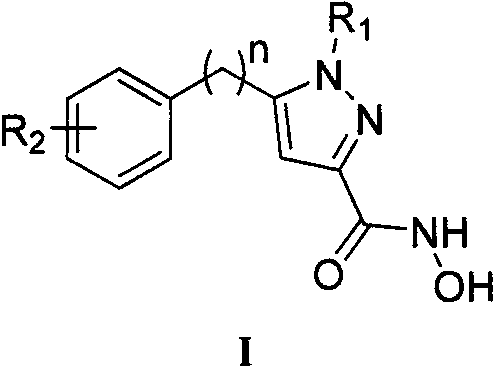
N-hydroxyl-5-substituted-1H-pyrazol-3-formamide compound as well as preparation method and use thereof
A technology of compounds and substituents, applied in the field of medicinal chemistry, can solve problems such as inapplicability, poor membrane permeability, and poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
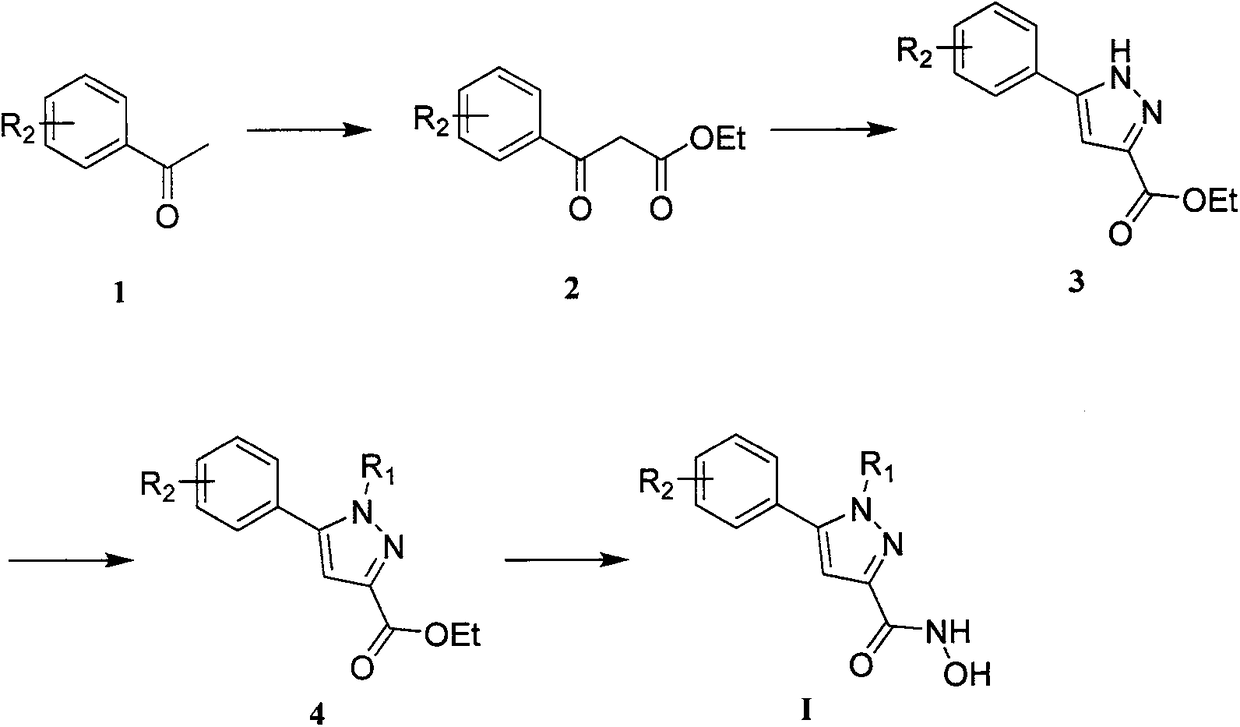
[0024] Example 1 Synthesis of some compounds of the present invention.
[0025] Preparation of ethyl 5-phenyl-1H-pyrazole-3-carboxylate:
[0026]
[0027] Add 383mg (16.7mmol) sodium metal in batches to 15mL absolute ethanol, stir at room temperature until dissolved, then add diethyl oxalate (1.28g, 8.75mmol). Dissolve 1g (8.33mmol) of acetophenone in 15mL of absolute ethanol, and add it dropwise to the freshly prepared sodium ethoxide solution under ice-cooling, return to room temperature after the dropwise addition, stir for 6 hours, add 15mL of 15% dilute hydrochloric acid to acidify, Subsequently, most of the ethanol was removed by rotary evaporation, diluted with 10 mL of water, extracted three times with 100 mL of ethyl acetate, and the organic phases were combined and dried over anhydrous sodium sulfate. The ethyl acetate in which the product was dissolved was directly spin-dried to obtain the crude product of the intermediate, which was re-dissolved by adding 15 mL...
Embodiment 2
[0052] Example 2 Experiment of compounds inhibiting acid sphingomyelinase activity.
[0053] Acid sphingomyelinase can hydrolyze sphingomyelin in cells to generate ceramide. For a certain amount of fluorescently labeled reaction substrates, different enzyme activities catalyze and generate different amounts of products. By detecting the content of products, the level of enzyme activity can be investigated. The present invention carries out experimental design according to this principle. Extract the protein in the cultured cells, add buffer, fluorescently labeled reaction substrate, and then add different concentrations of compounds respectively, set up a blank control group, perform fluorescence analysis after the reaction, and finally calculate the IC of the compound 50 value.
[0054] The specific results are shown in the table:
[0055] Table 1: Acid sphingomyelinase inhibitory activity of some compounds of the present invention
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com