Preparation method of sitafloxacin hydrate
A technology of sitafloxacin and compounds, applied in the field of preparation of sitafloxacin, can solve the problems of long steps, low product yield, easy formation of isomer impurities, etc., and achieve simple steps and low content of isomer impurities , reducing the effect of the reaction step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
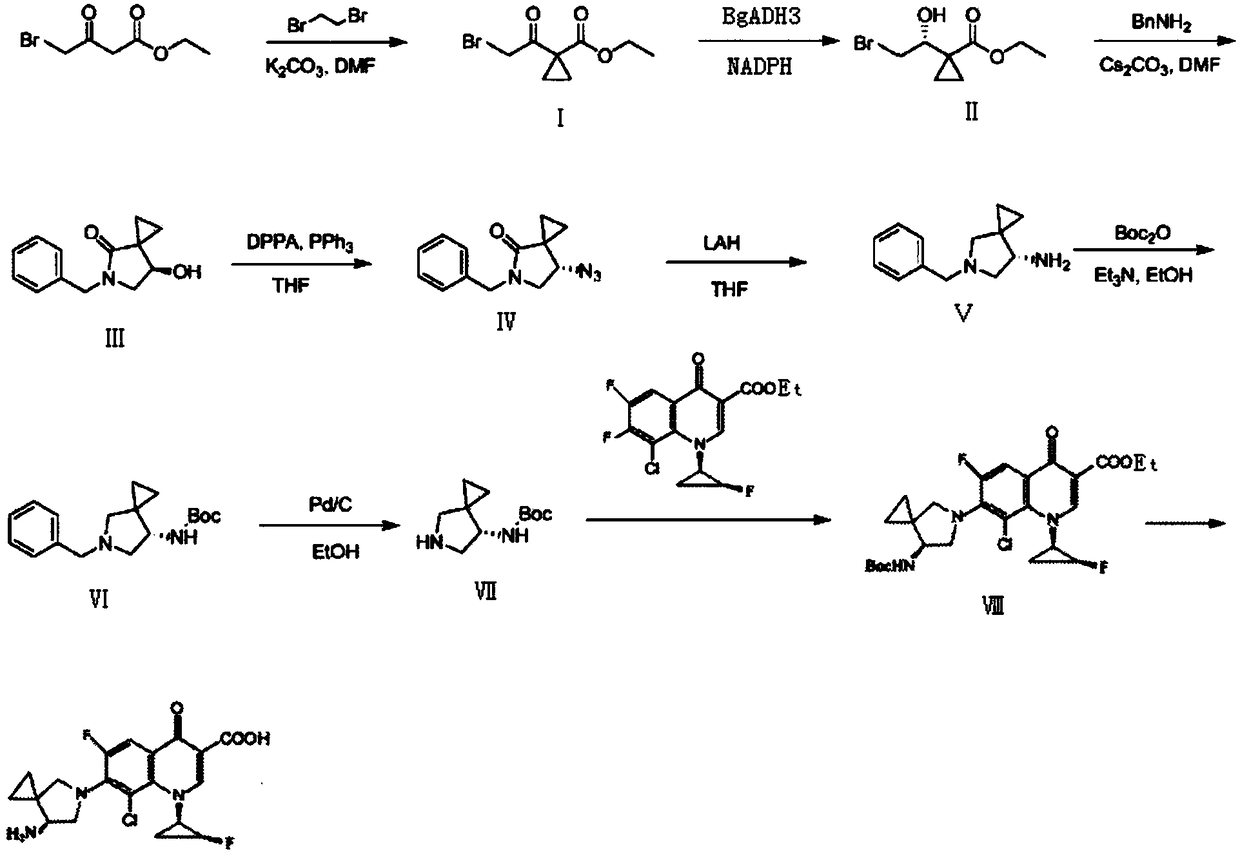
[0027] Preparation process of sitafloxacin:
[0028] step 1,
[0029] Put 21g of ethyl 4-bromoacetoacetate in a reaction flask, add 120mL of DMF, 37.6g of 1,2-dibromoethane, and 52.5g of potassium carbonate in sequence, control the temperature of the reaction solution at 25-30°C, and react for 20- After 30 hours, after the reaction was completed, it was added to 600 mL of water, extracted with ethyl acetate, concentrated and distilled to dryness to obtain 20.1 g of compound I with a yield of 85.5%.
[0030] Step 2,
[0031] Take 20g of compound I, add 400mL of methyl tert-butyl ether to dissolve, take 12g of carbonyl reductase BgADH3, 2g of NADPH, and 20g of glucose in 200mL of deionized water, mix the two-phase solvents, control the reaction temperature at 40°C, and react for 5-6 hours under stirring After the reaction was completed, the organic phase was separated, concentrated and distilled to dryness to obtain 19.2 g of compound II with a yield of 95.2% and a purity of 9...
Embodiment 2
[0048] Preparation process of sitafloxacin:
[0049] step 1,
[0050]Put 420g of ethyl 4-bromoacetoacetate in a reaction flask, add 2.4L of DMF, 750g of 1,2-dibromoethane, and 1.05kg of potassium carbonate in sequence, control the temperature of the reaction solution at 25-30°C, and react for 20- After 30 hours, after the reaction was completed, it was added to 12 L of water, extracted with ethyl acetate, concentrated and distilled to dryness to obtain 391 g of compound I with a yield of 82.9%.
[0051] Step 2,
[0052] Take 300g of compound I, add 6L of methyl tert-butyl ether to dissolve, take 180g of carbonyl reductase BgADH3, 30g of NADPH, and 300g of glucose, dissolve in 3L of deionized water, mix the two-phase solvents, control the reaction temperature at 40°C, and react for 5-6 hours under stirring After the reaction was completed, the organic phase was separated, concentrated and distilled to dryness to obtain 287 g of compound II with a yield of 94.9% and a purity o...
Embodiment 4
[0072] Carbonyl Reductase Selective Reduction of Carbonyl Comparative Example
[0073] Take 20 g of compound I prepared in Example 1, add 400 mL of n-hexane to dissolve, take 12 g of carbonyl reductase BgADH3, 2 g of NADPH, and 20 g of glucose and dissolve them in 200 mL of deionized water, mix the two-phase solvents, control the reaction temperature at 40°C, and react for 5 -6 hours, after the reaction was completed, the organic phase was separated, concentrated and distilled to dryness to obtain 17.8 g of compound II with a yield of 88.3% and an HPLC purity of 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com