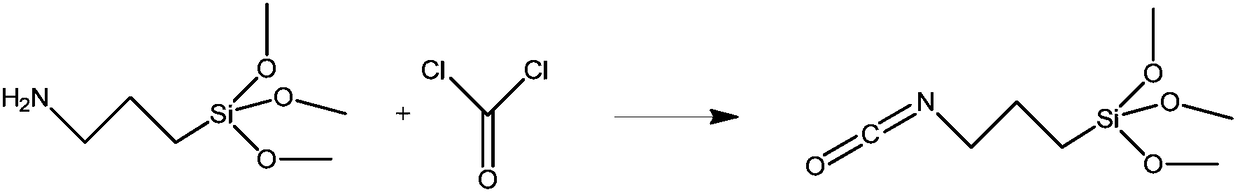
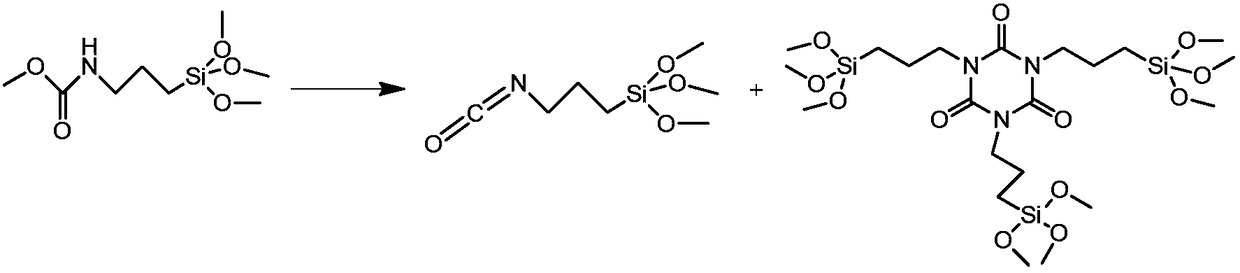
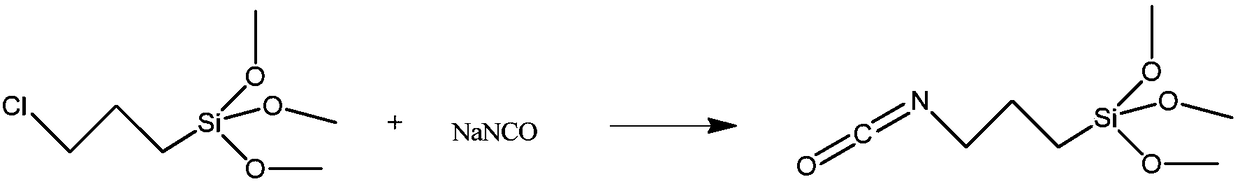
Method for preparing 3-isocyanatopropyltrimethoxysilane
A technology of esteryl propyltrimethoxysilane and trimethoxysilane, which is applied in the field of preparation of 3-isocyanatopropyltrimethoxysilane, and can solve problems such as difficult control of by-products, harsh reaction conditions, and long reaction time , to achieve the effects of safe production, easy control, good product quality and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 126 g of methyl [3-(trimethoxysilane)propyl] carbamate, 59 g of triethylamine and 400 ml of toluene into a 1 L reaction kettle, and stir. Under nitrogen protection, add 45g of methyltrichlorosilane dropwise at 20-40°C, after the dropwise addition, slowly raise the temperature to 95-100°C, react for 1h, take samples for analysis, [3-(trimethoxysilane)propyl]carbamic acid When the GC content of methyl ester is less than 1%, the heating can be stopped. Cool down to 20-30°C and filter, and wash the filter cake twice with 100ml of toluene. The filtrates were combined, toluene was concentrated, and 107 g of the product was evaporated under reduced pressure, with a yield of 96% and a content of 98%.
Embodiment 2
[0035] Put 150 g of [3-(trimethoxysilyl)propyl]methyl carbamate, 96 g of triethylamine and 400 ml of toluene into a 1L reaction flask, and stir. Under nitrogen protection, add 75g of methyltrichlorosilane dropwise at 20-40°C, after the dropwise addition, slowly raise the temperature to 95-100°C, react for 1h, take samples for analysis, [3-(trimethoxysilane)propyl]carbamic acid When the GC content of methyl ester is less than 1%, the heating can be stopped. Cool down and filter, and the filter cake is washed twice with 150ml of toluene. The filtrates were combined, the solvent was evaporated, and 127 g of the product was evaporated under reduced pressure, with a yield of 95.8% and a content of 98%.
Embodiment 3
[0037] Add 169 g of [3-(trimethoxysilane)propyl] methyl carbamate, 111 g of triethylamine and 400 ml of toluene into a 1 L reaction kettle, and stir. Under nitrogen protection, add 106g of methyltrichlorosilane dropwise at 20-40°C, after the addition is complete, slowly raise the temperature to 95-100°C, react for 1h, take samples for analysis, [3-(trimethoxysilane)propyl]carbamic acid When the GC content of methyl ester is less than 1%, the heating can be stopped. Cool down and filter, and the filter cake is washed twice with 150ml of toluene. The filtrates were combined, the solvent was concentrated, and 142 g of the product was evaporated under reduced pressure, with a yield of 95.3% and a content of 97.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com