Immobilization of pyruvate oxidase
A technology of pyruvate oxidase and pyruvate oxidase enzyme liquid, which is applied in the field of immobilization of pyruvate oxidase, can solve the problems of difficult purification, easy inactivation, and non-reusability, and achieve high enzyme activity and tolerance Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
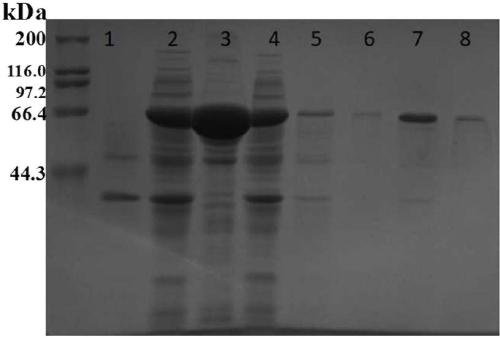
[0028] Example 1: Expression and purification of pyruvate oxidase gene
[0029] According to the sequence (sequence ID EF017806.1) reported on Genbank derived from the gene of A. viridans, it was sent to GenScript Company to synthesize the target gene. The cloning vector was pET-28a, and the restriction site was EcoR 1, xho 1.
[0030] Transform the pET-28a(+) recombinant plasmid containing the target gene of pyruvate oxidase into the expression host bacteria E. coli BL21(DE3) (NBE, Cat NO.C2527H) obtained recombinant microorganism E. coli BL21(DE3)- pET-28a(+), spread the recombinant microorganism on a plate containing 50 mg / L kanamycin and 24 mg / L IPTG, culture it at 37 ℃ for 16-20 h, pick a single The colony was transferred to the colony PCR, and the gene sequence was verified to be correct by sequencing.
[0031] The obtained positive clones were cultured in LB medium at 37 °C until the OD600nm was between 0.5-0.6, and IPTG was added to a concentration of 0.2 mM, ...
Embodiment 2
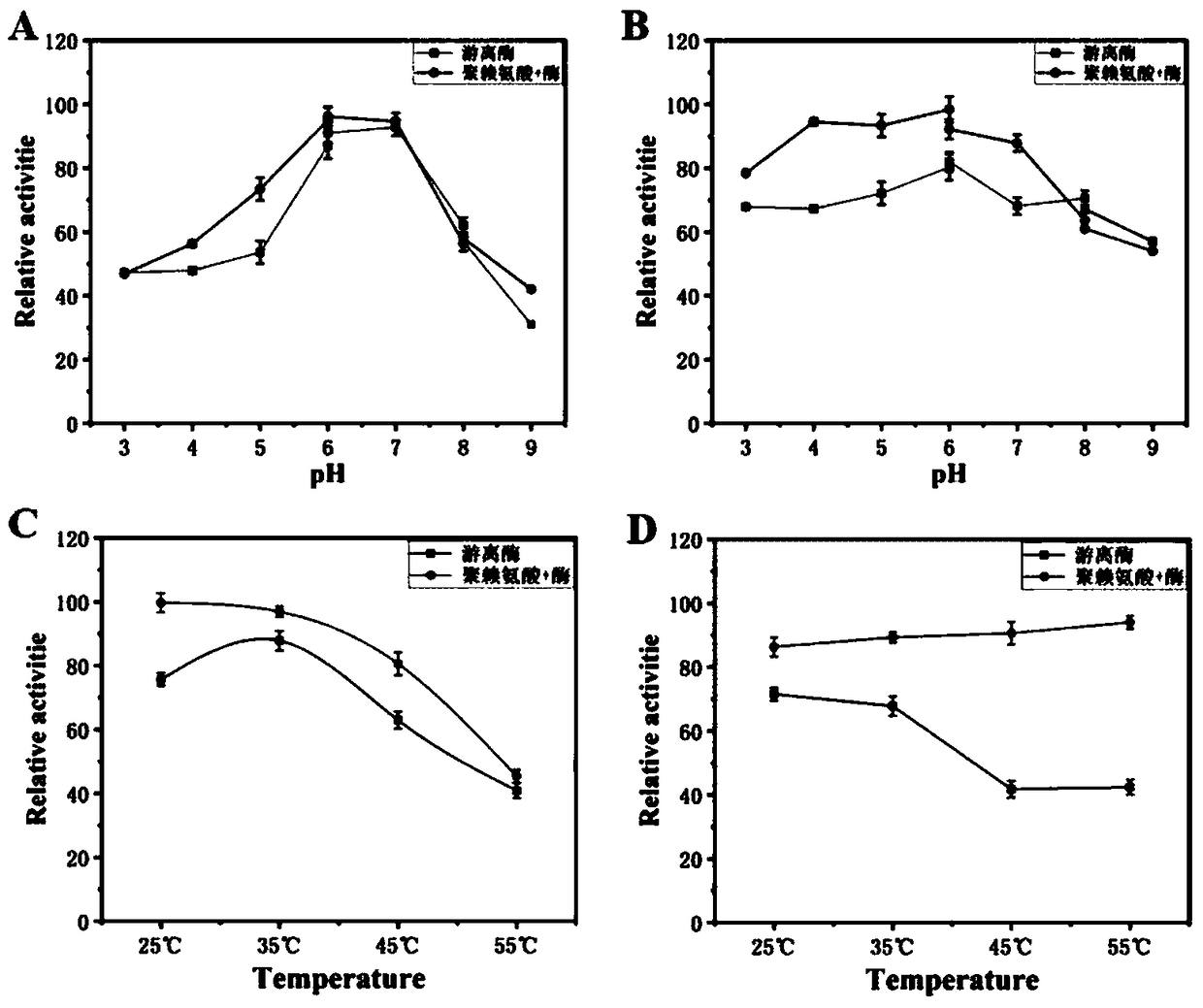
[0033] Example 2: Immobilization of pyruvate oxidase
[0034] (1) Measure the enzyme activity of the pyruvate oxidase pure enzyme obtained in Example 1, aliquot and ensure that each tube contains 1 U of pyruvate oxidase enzyme activity, and store it at -20°C for future use.
[0035] (2) Using the modification method of drop coating layer by layer, place the cleaned Pb electrode in 10% HNO 3 and 2.5% of K 2 Cr 2 o 7 , using cyclic voltammetry to scan a circle for electrochemical oxidation treatment;
[0036] (3) Take 6 µL of freshly prepared 30 mmol / L carbodiimide solution (EDC, N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride) and titrate it on the surface of the oxidized Pb electrode to fix EDC on the electrode surface , dry at room temperature;
[0037] (4) Titrate 6 µL of the prepared poly-lysine solution with a mass concentration of 0.18% on the Pb electrode, and the EDC and poly-lysine have a cross-linking effect, so that poly-lysine forms a firm modificat...
Embodiment 3
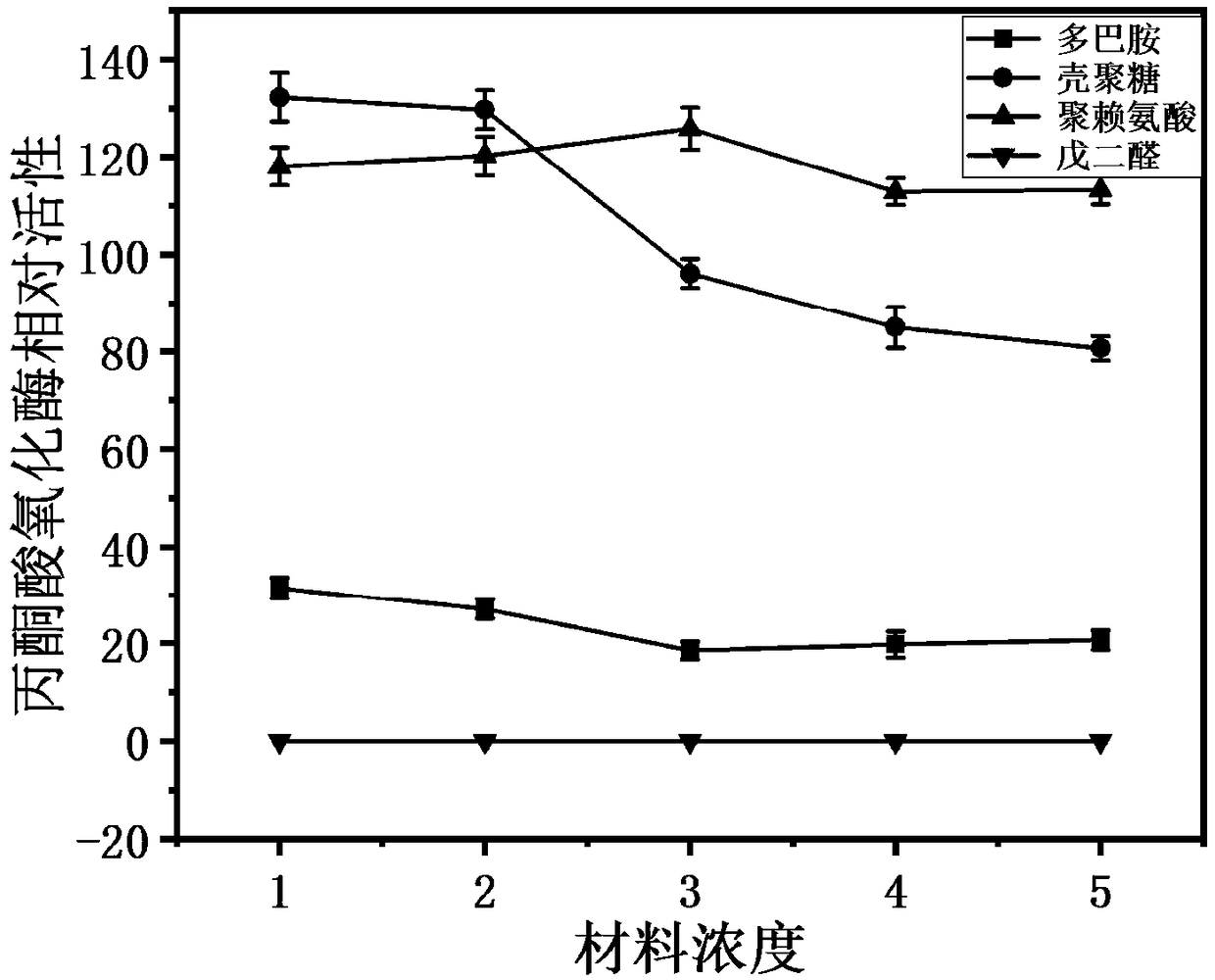
[0046] In this embodiment, glutaraldehyde, dopamine, and chitosan carriers are used as comparative examples to illustrate the technical effect of pyruvate oxidase immobilization.
[0047] (1) Measure the enzyme activity of the obtained pyruvate oxidase pure enzyme, aliquot and ensure that each tube is 1U of pyruvate oxidase enzyme activity, and mix the enzyme solution with different concentrations of glutaraldehyde solution (0.125%, 0.25% , 0.375%, 0.5%, 0.625%) were mixed according to a 1:1 dilution ratio, and titrated on the surface of the Pb electrode by titration, then the electrode was placed in a ventilated place at room temperature to dry for 18 hours, and the enzyme activity was tested after washing the electrode surface with distilled water.
[0048] (2) Measure the enzyme activity of the obtained pyruvate oxidase pure enzyme, aliquot and ensure that each tube is 1 U of pyruvate oxidase enzyme activity, and divide the enzyme solution into the dopamine solution prepared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com