N-(2-(substituted-naphth-1-yl)ethyl)-substituted amide compounds, and preparation thereof and application thereof
A compound, the technology of acetamide, which is applied in the field of N-(2-(substituted-naphthalen-1-yl)ethyl) substituted amide compounds, its preparation and application, can solve individual differences and poor oral bioavailability And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
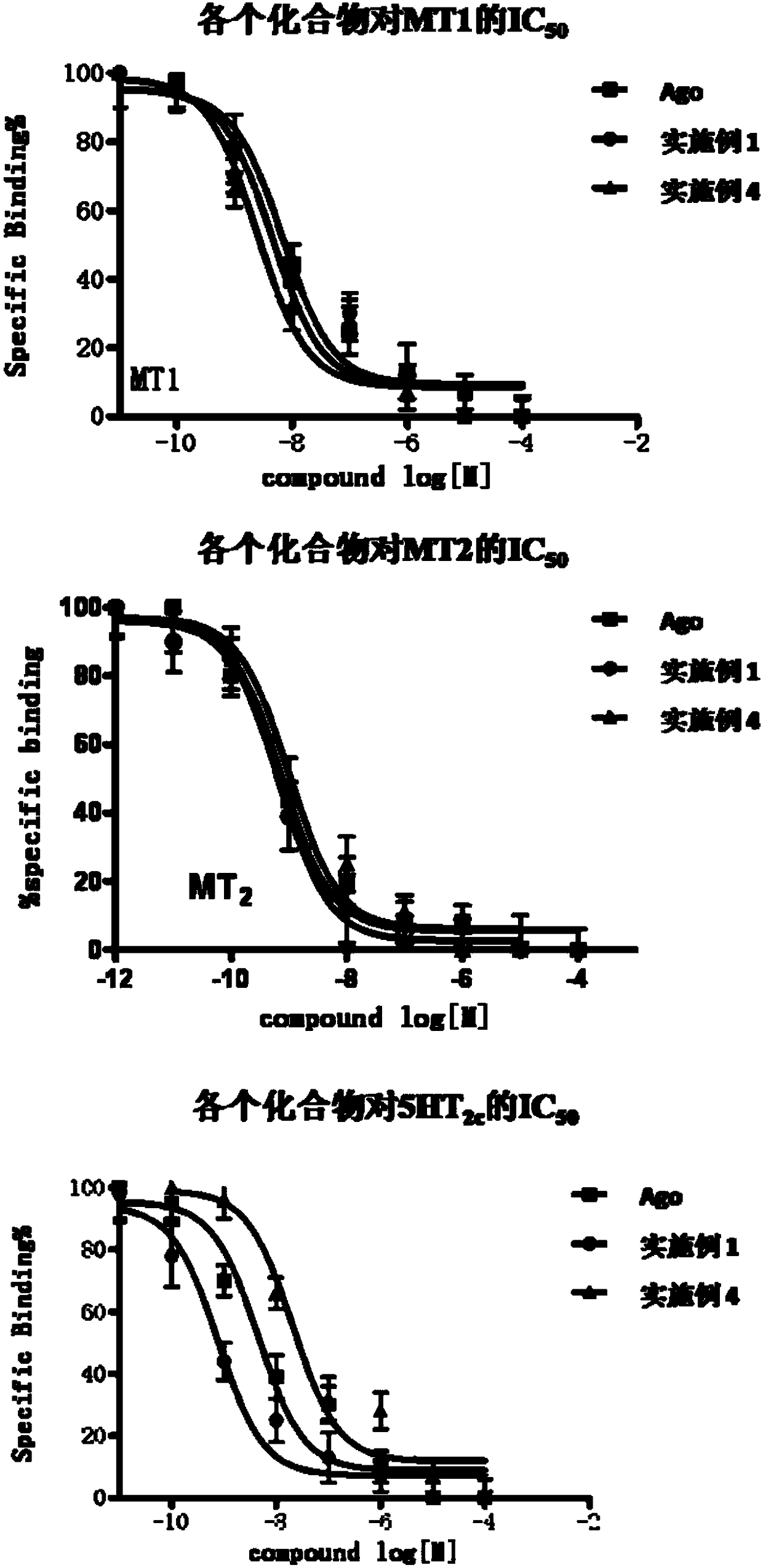
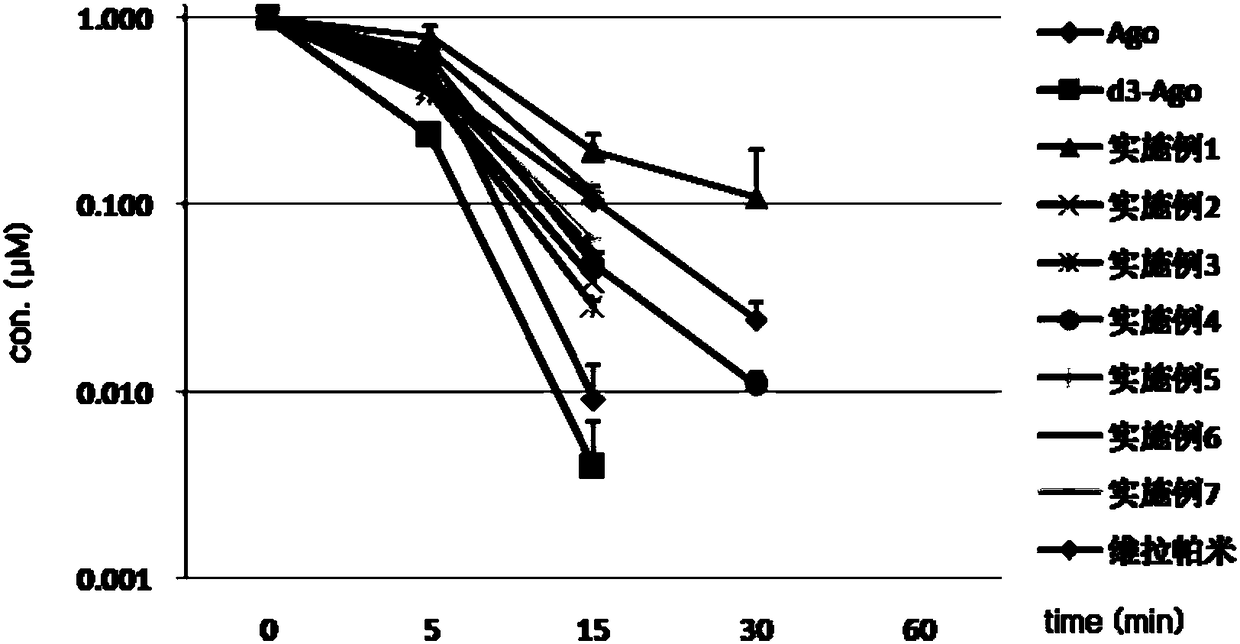
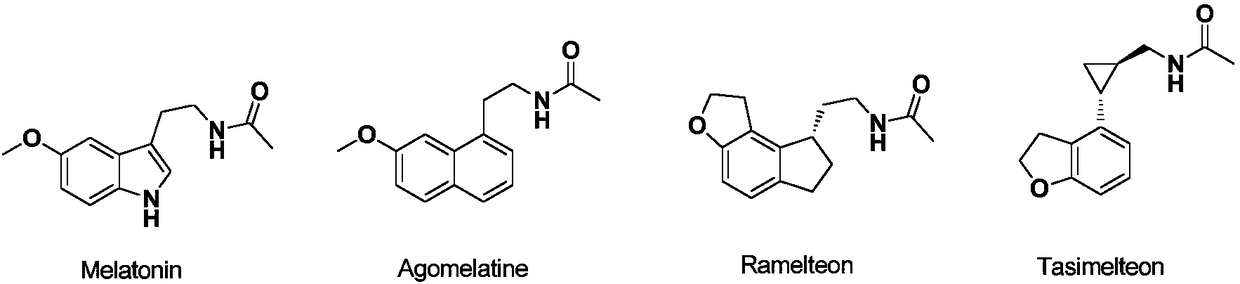
Image
Examples
preparation example Construction
[0123] In one embodiment of the present invention, the synthetic method of the present invention also comprises:
[0124] The above-mentioned acetamide / propionamide compound is dissolved in toluene and reacted with anhydrous aluminum chloride to obtain naphthol; and
[0125] Reaction of the naphthol with deuterated iodomethane yields a deuterated acetamide / propionamide compound.
[0126] In one embodiment of the present invention, the synthetic method of the present invention also comprises:
[0127] The acetamide / propionamide compound or deuterated acetamide / propionamide compound described above is reacted with an acid to form a salt.
[0128] For specific operations, for example, reference may be made to the description of the embodiments.
[0129] According to the detailed teaching of the present invention and the existing general knowledge of synthesis, those skilled in the art can easily synthesize the compound of formula I of the present invention.
[0130] Another as...
Embodiment 1 and Embodiment 2、8
[0141] The synthesis of embodiment 1 and embodiment 2,8:
[0142] The reaction scheme is as above, and the specific synthesis operation is as follows:
[0143] Intermediate 1:
[0144]
[0145] 3-liter three-necked bottle, with a condenser tube on the shelf. Add 328 grams (2.46 mol) of aluminum trichloride into 1.7 liters of dichloromethane under an ice bath, and stir to dissolve it completely. Add 156.8 g (138 mL, 1.10 mol) of o-chloroanisole at one time, and then add 123 g (1.23 mol) of succinic anhydride in batches, the system will generate heat and gas, and spontaneous reflux will occur. After the spontaneous reflux is over, start heating to reflux. After refluxing for 2 hours, the progress of the reaction was monitored by TLC, and the reaction was terminated when the starting material disappeared. After natural cooling, pour into 5 liters of ice water. Under stirring, it was acidified with 300 ml of concentrated hydrochloric acid, and a large amount of solids ap...
Embodiment 1
[0171] Example 1: N-(2-(6-Chloro-7-deuteromethoxy-naphthalen-1-yl)ethyl)acetamide
[0172]
[0173] 17.3 grams of intermediate 7 were dissolved in 1 liter of acetone, 27.2 grams of potassium carbonate, 1.3 grams of potassium iodide, 12.4 grams (5.31 milliliters) of deuterated methyl iodide were added thereto, after 24 hours of reaction at room temperature, TLC monitored the reaction to be complete, filtered, and the filtrate was spin-dried , add 400 milliliters of ethyl acetate to the residue, then wash with 200 milliliters of water and saturated aqueous sodium chloride successively, filter the organic phase after drying with anhydrous sodium sulfate, add 2 grams of gacs to the filtrate and boil for 30 minutes, then filter, Spin-dried and treated with cyclohexane / ethyl acetate to obtain a white powdery solid. Yield 81.4%, melting point: 148-151°C
[0174] 1 H-NMR (400MHz, DMSO-d6), δ: 1.83 (s, 3H), 3.11-3.20 (m, 2H), 3.29-3.35 (m, 2H), 7.30-7.36 (m, 2H), 7.70-7.72 (dd,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com