Preparation method of self-supporting nanometer porous silver electrode
A nanoporous silver, self-supporting technology, applied in nanotechnology, nanotechnology, nanotechnology and other directions for materials and surface science, can solve the problem of high process energy consumption, achieve low energy consumption, reduce energy consumption, oxygen reduction Activity enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
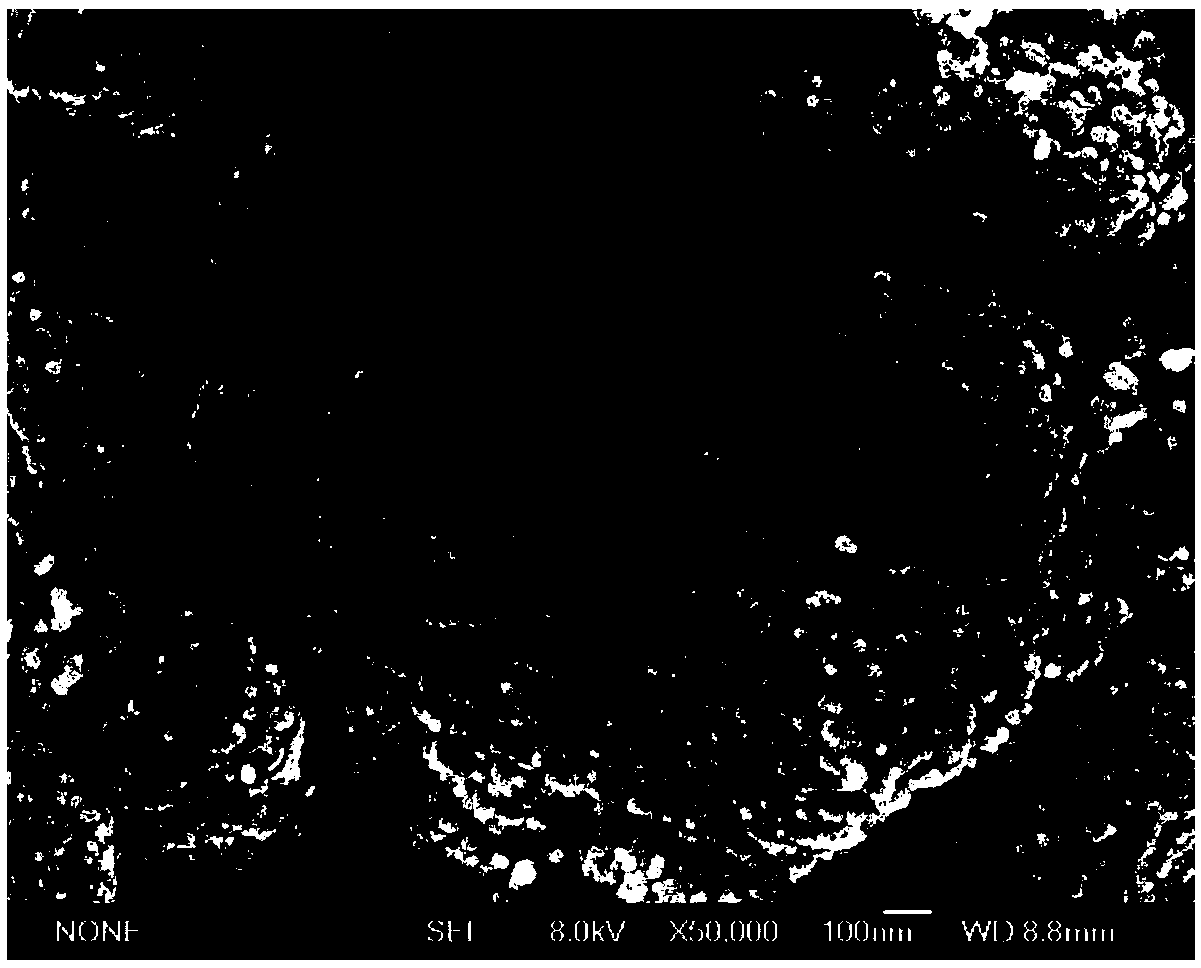
[0021] After polishing the Ag electrode with a diameter of 5 mm to the mirror surface with 50 nm alumina abrasive, the surface of the electrode was cleaned with deionized water, and then ultrasonicated in ethanol and ultrapure water for 15 s, respectively. Put the polished Ag electrode into the solution containing 10mM Cu 2+ Sodium chloride electrolyte, rinse with water after soaking for 10 minutes. The soaked Ag electrode is used as the working electrode, the Pt wire is used as the counter electrode, and the Hg / HgO is used as the reference electrode. The electrolyte is 0.1M NaOH. -1 Carry out cyclic voltammetry scan (20 circles) at the scan rate of 1000°C, electrochemically reduce the electrode to generate nanoporous silver, and carry out electron microscope scanning to the Ag electrode after reduction, the result is as follows figure 1 shown.
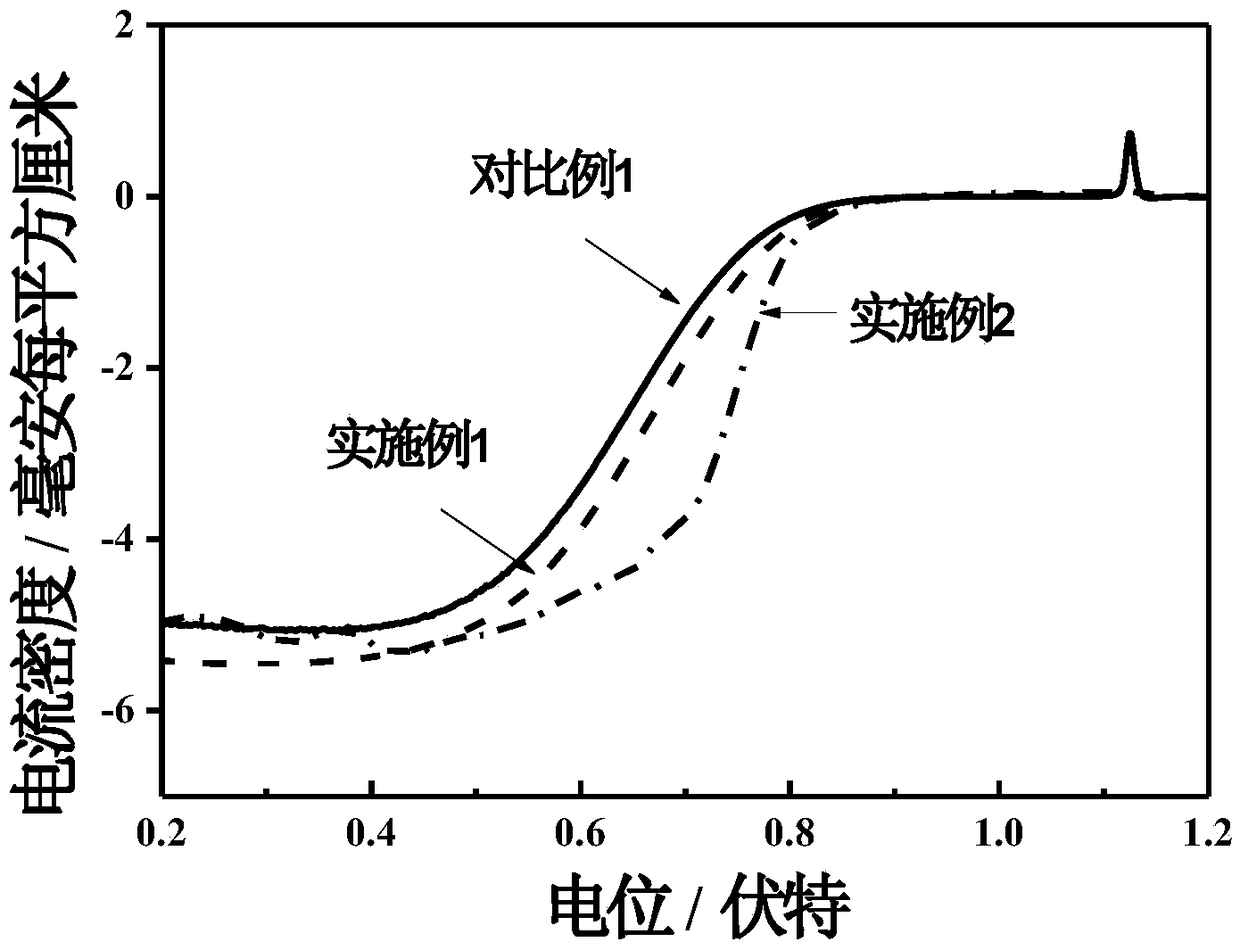
[0022] The ORR polarization curve was tested at an electrode rotation speed of 1600rpm, and the ORR half-wave potential was 0.672V...
Embodiment 2
[0024] After polishing the Ag electrode with a diameter of 5 mm to the mirror surface with 50 nm alumina abrasive, the surface of the electrode was cleaned with deionized water, and then ultrasonicated in ethanol and ultrapure water for 15 s, respectively. Put the polished Ag electrode into the solution containing 0.1M Fe 3+ Sodium chloride electrolyte, rinse with water after soaking for 10 minutes. The soaked Ag electrode is used as the working electrode, the Pt wire is used as the counter electrode, and the Hg / HgO is used as the reference electrode. The electrolyte is 0.1M NaOH. -1 Cyclic voltammetry scanning (20 circles) is carried out at the scanning speed, and the electrode is electrochemically reduced to generate nanoporous silver.
[0025] The ORR polarization curve was tested at an electrode rotation speed of 1600rpm, and the ORR half-wave potential was 0.732V, which was 60mV higher than that of the initial Ag electrode, as shown in figure 2 shown.
Embodiment 3
[0027] The Ag wires with a diameter of 0.5 mm were placed in deionized water and acetone and ultrasonically cleaned for 30 min, and then the surface of the Ag wires was cleaned with deionized water. Put the ultrasonically treated Ag wire into 0.1M Cu 2+ Potassium chloride electrolyte solution, rinse with water after soaking for 10 minutes. The soaked Ag wire is used as the working electrode, the Pt wire is used as the counter electrode, and the Hg / HgO is used as the reference electrode. The electrolyte is 0.1M NaOH. -1 A cyclic voltammetry scan (20 circles) was carried out at a scan rate of 100%, and the electrode was electrochemically reduced to generate nanoporous silver.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com