Novel crystal of sacubitril valsartan sodium as well as preparation method and application thereof
A technology of sacubitril valsartan and sartan sodium, which is applied in the field of new crystal form of sacubitril valsartan sodium and its preparation, can solve problems such as poor compressibility, difficulty in direct tablet compression, poor fluidity, etc., and achieve shortening Production time, improvement of hygroscopicity, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 preparation approach ( reference CN200680001733.0 Embodiment 2
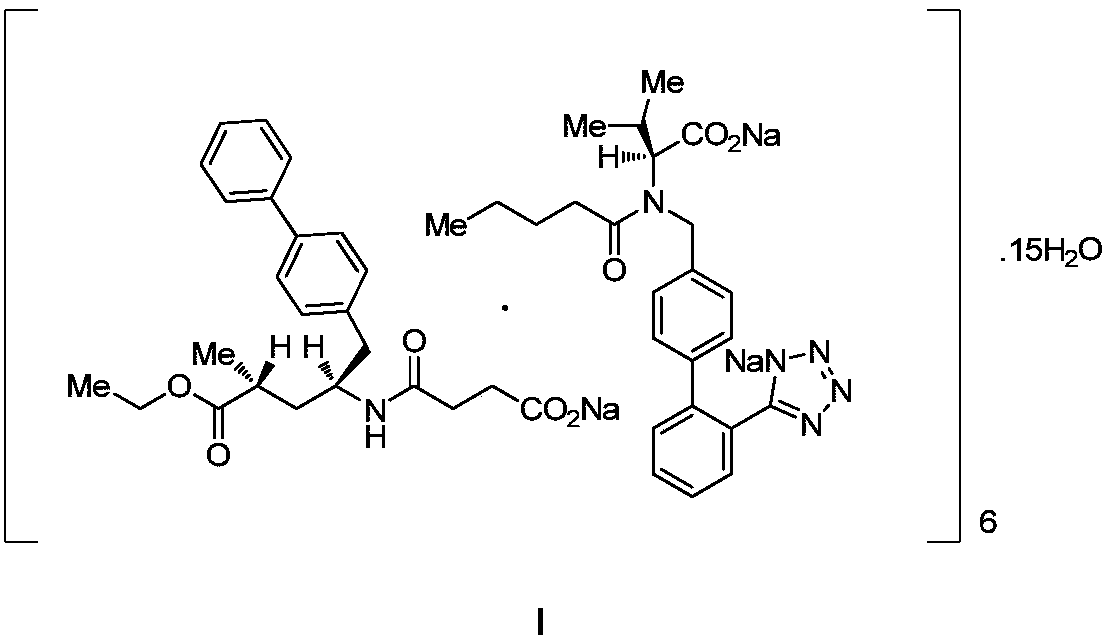
[0038] In a 500mL flask, add 9.45g of sacubitril (22.96mmol), 10.0g of valsartan (22.96mmol) and 300mL of acetone, stir to dissolve, add dropwise sodium hydroxide aqueous solution (2.76g, namely 68.90mmol, dissolved in 8mL water), stirred at 20-25°C for 2h. Concentrate under reduced pressure at 15-30°C to a volume of 150 mL. Add 150 mL of isopropyl acetate, and concentrate under reduced pressure at 15-30°C to a volume of 150 mL. Add 150 mL of isopropyl acetate, and concentrate under reduced pressure at 15-30°C to a volume of 150 mL. Stir for 1 h at 20-25°C. Suction filtration, washing with isopropyl acetate, and vacuum drying of the solid at 30-35°C gave 19.51 g of sacubitril-valsartan sodium.
Embodiment 2
[0039] Example 2 Preparation method of sacubitril valsartan sodium crystal form Y
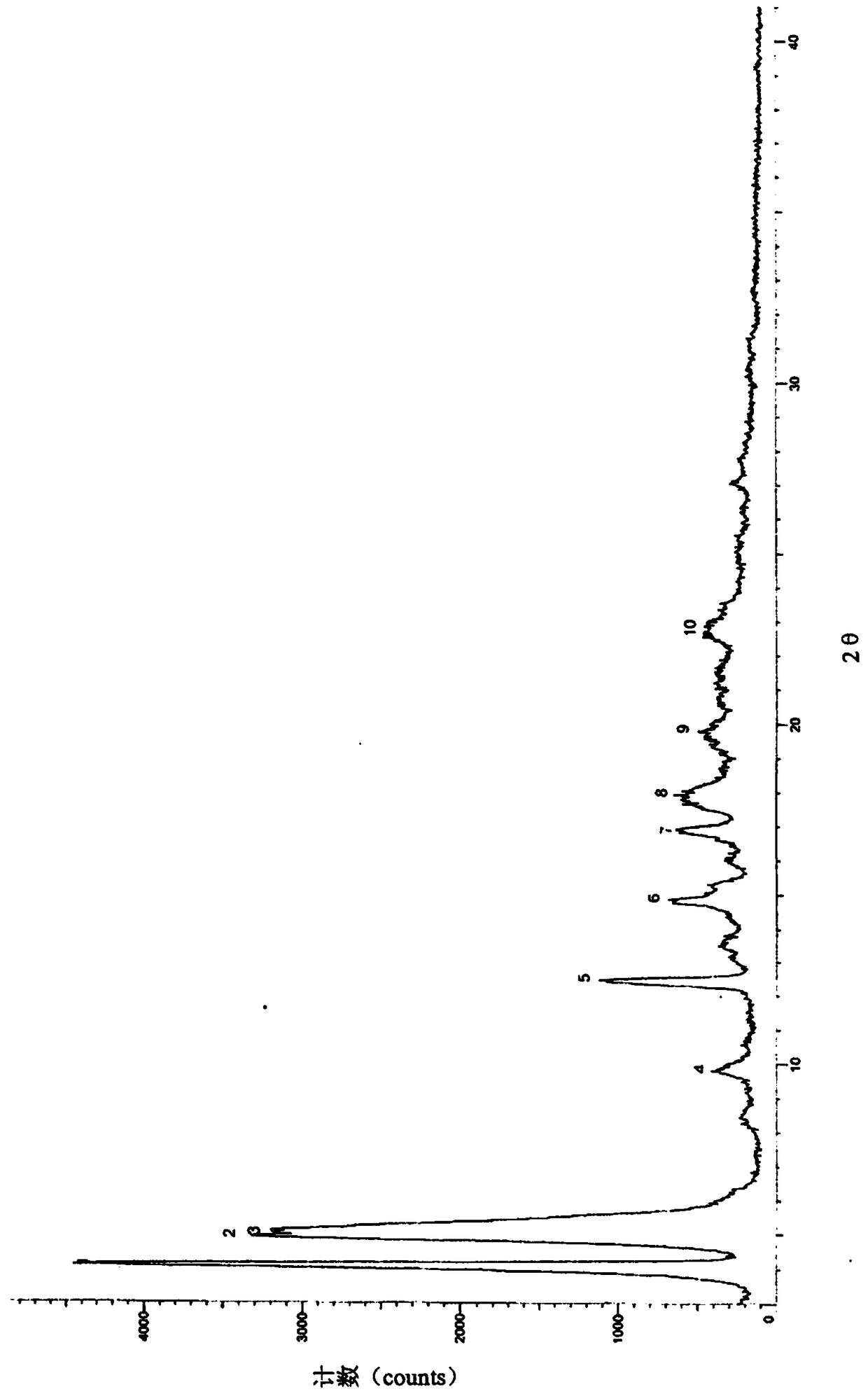
[0040] Add 1 g of sacubitril-valsartan sodium (1.04 mmol, prepared in Example 1), 1 mL of water and 10 mL of ethanol to a 100 mL three-necked flask, stir at room temperature for 1 h, and concentrate to dryness under reduced pressure. Add 8 mL of acetone and 30 mL of methyl tert-butyl ether, and stir at 50°C for 2 hours. Cool down to 10°C and stir for 1h. Suction filtration, washing with methyl tert-butyl ether, and vacuum drying gave 0.908g off-white solid, yield 90.8%, HPLC purity 99.8%, all simple impurities were less than 0.1%, XRD detected as a crystalline solid (see attached figure 1 ).
[0041] Its X-ray diffraction data are shown in the following table:
[0042] serial number
Embodiment 3
[0043] Example 3 : Preparation of existing crystal forms (CN201580002782.5 Crystal Form II, I, CN201510618916.8 Crystal Form A, WO2016151525 Form I)
[0044] Preparation of CN201580002782.5 Crystal Form II: In a 250mL reaction bottle, dissolve 667mg of sacubitril-valsartan sodium in 10mL of methanol and 100mL of toluene, filter, seal with a pinhole-punched parafilm, and volatilize at room temperature. Suction filtration to obtain sacubitril valsartan sodium crystal form II.
[0045] CN201580002782.5 Preparation of crystal form I: Suspend 205 mg of sacubitril-valsartan sodium crystal form II above in 1 mL of cumene and stir at 50°C for 2 days to obtain sacubitril-valsartan sodium crystal form I .
[0046] Preparation of CN201510618916.8 Form A: 2.0 g (4.6 mmol) of valsartan and 1.88 g (4.6 mmol) of sacubitril were dissolved in absolute ethanol (10 mL) at room temperature. Add 0.55 g (13.8 mmol) of sodium hydroxide in 1 mL of an aqueous solution, raise the temperature to 40°...
PUM
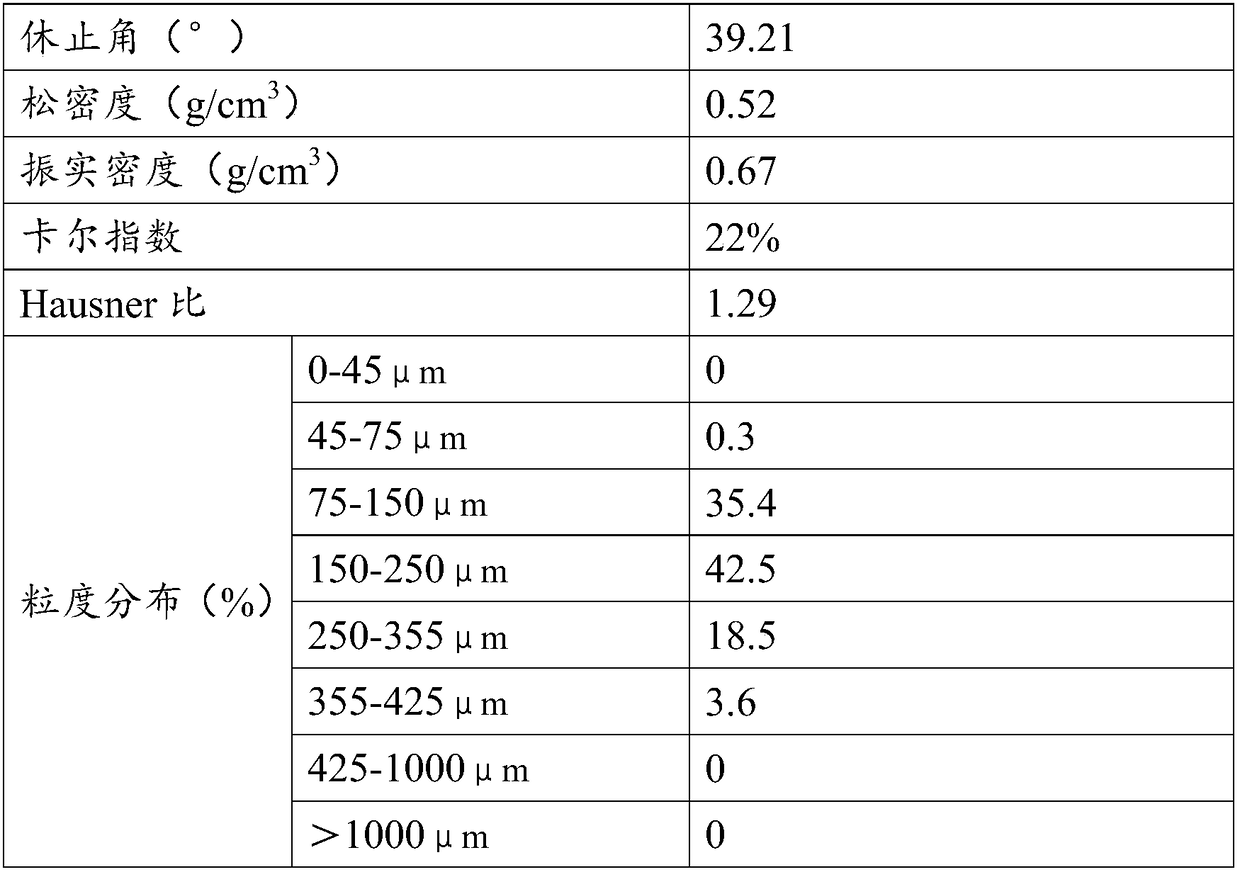
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com